CLINICAL ENDOSCOPY/MULTIPHOTON IMAGING/FIBER OPTICS: Multiphoton endoscopy for clinical applications
DAVID M. HULAND, DAVID R. RIVERA, DIMITRE G. OUZOUNOV, and CHRIS XU
A medical test commonly used in the diagnosis of various diseases, including most cancers, is the tissue biopsy. Done by a surgeon or interventional radiologist, this procedure involves the removal of a small sample of tissue from a suspicious site in the body of a patient. The tissue is processed into histopathology slides, frequently using the hematoxylin and eosin stain (H&E), and analyzed by a pathologist.
While the procedure defines the current standard of care and is usually accurate in diagnosing and staging various cancers and other diseases, it is associated with several disadvantages. Among these are high procedural cost, patient discomfort, and complications related to tissue removal. In cases where the suspicious site is not easily identified, there is also risk of removing tissue from the wrong area, meaning that the patient must return for repeated biopsy procedures. In addition, the time to diagnosis is frequently on the order of several days to weeks, causing psychological discomfort to the patient.
Thankfully, new technology promises to improve the situation for both doctors and patients.
in vivo multiphoton microscopy (MPM) has become a valuable tool for the study of structures located deep within intact animals.1 Specifically, two-photon fluorescence (TPF) and second-harmonic generation (SHG) enable imaging of unprocessed and unstained biological tissues. These label-free techniques can immediately produce high-resolution images comparable to standard histology, and they show great promise for medical diagnostics of numerous diseases.2-4 The maximum imaging depth, however, is limited in most tissues to less than 2 mm. Therefore, for MPM to be viable as a clinical tool, it is critical to develop miniaturized TPF and SHG microscopes that operate as endoscopes.
Multiphoton endoscopes (MPE) could provide real-time margin assessment during tumor resection and guide conventional biopsy procedures. Many groups have contributed significantly toward these goals.5-9 This article summarizes the work and progress of researchers at Cornell University towards the goal of multiphoton endoscopes for real-time tissue diagnosis in a clinical setting.
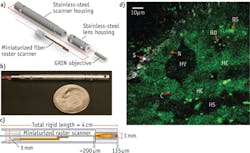
A clinically useful MPE needs to achieve multiple imaging parameters—e.g., high resolution, fast frame rates, large field of view (FOV), axial sectioning—within a compact device. Our efforts have produced a compact, flexible MPE capable of acquiring sub-micron-resolution, in vivo images of unstained tissues (see Fig. 1).10,11 The device consists of a miniaturized resonant/nonresonant fiber raster scanner and a gradient index (GRIN) lens assembly packaged in a rigid tube with a 3 mm outer diameter and a length of 4 cm. It can acquire 110 × 110 μm multiphoton images with high scan uniformity at 4 frames/s (512 × 512 pixels), and achieves lateral and axial resolutions for two-photon imaging of 0.8 and 10 μm, respectively. We demonstrated this device in in vivo imaging experiments in rats (see Fig. 1b).
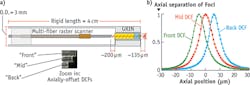
To help translate our endoscopes to the clinic, we demonstrated new techniques to overcome challenges such as motion artifacts, and to meet usage requirements. For instance, to achieve instantaneous axial sectioning and fast frame rates without sacrificing signal-to-noise ratio (SNR) per frame, we integrated parallel image acquisition.12 Multiphoton images are obtained simultaneously at three axial depths by incorporating three offset double-clad optical fibers (DCFs) into the miniaturized scanner, and subsequently packaging the multi-fiber scanner into the same endoscope housing (see Fig. 2).To achieve a larger image (FOV) while maintaining high spatial resolution, we integrated a lensed fiber into our miniaturized scanner.13 This reduced the fiber's output beam size, and increased FOV to a diameter of 440 μm while maintaining a one-photon lateral resolution of 1.1 μm.
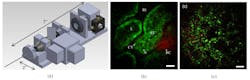
We also designed and tested a rigid endoscope with an outside dimension similar to that of a needle biopsy. We explored the use of long GRIN lens systems, and verified the possibility of MPM imaging through lenses up to 28.5 cm. We designed a portable, hand-held multiphoton microscope able to incorporate GRIN lens systems, and demonstrated it using an 8-cm-long, 1-mm-diameter doublet GRIN system.14 The resulting rigid MPE device acquires multiphoton images with a ~200-μm-diameter FOV at 4 frames/s (512 × 512 pixels), and achieves lateral and axial resolutions for two-photon imaging of 0.85 and 7.4 μm, respectively. We applied the device for in vivo imaging experiments in rats (see Fig. 3a and 3b).One advantage of this system is that it is potentially compatible with several other imaging techniques. For example, we explored three-photon endoscopy using a convenient femtosecond fiber laser at ~ 1040 nm.15 Previous studies have indicated that phototoxicity and photodamage may be reduced when using longer excitation wavelengths.16,17 In addition, the longer excitation wavelength will improve MPE's tissue penetration depth.18-20 Resulting ex vivo images of mouse lung are shown in Fig. 3c.
Two modes better than one
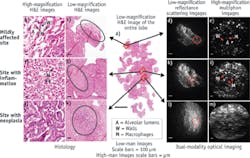
Testing of our prototypes highlighted the fact that without optical zoom, the devices would have limited application. The relatively small FOV makes examination of large areas of interest difficult and time-consuming, and more importantly inhibits the study of tissue architecture containing essential diagnostic information. By contrast, a low resolution/large FOV would allow a clinician to survey a large area, study tissue architecture, and identify sites of interest. Then, switching to high resolution/small FOV would enable examination of cellular detail at the sites of interest. For example, current pathology labs use at least two optical zooms, a 4x objective for architecture viewing, and a 20x (or 40x) objective for imaging cellular details.
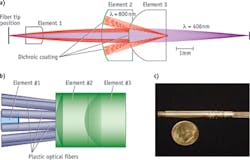
We designed, built, and tested a dual-modality endomicroscope that provides both large FOV through reflectance/scattering imaging and high spatial resolution through multiphoton imaging. The essential element of the device is a miniature (3 mm OD) catadioptric lens (see Fig. 4a) based on the principle of wavelength-division multiplexing.21 The variation of magnification and FOV is enabled by the dichroic coatings deposited at the proximal surfaces of two of the three elements, and is achieved by changing the wavelength of the excitation light without any mechanical adjustment at the distal end. Fluorescence signal is collected through large-mode-area plastic optical fibers located next to element #2 (see Fig. 4b). We integrated this varifocal lens with the previously demonstrated10 miniaturized resonant/ non-resonant fiber raster scanner into a fully functional endomicroscope probe22 with 5 mm OD and 5 cm rigid length (see Fig. 4c).With further improvements, we aim to enable par-focal zoom operation (overlapping of two magnification planes), and to combine high-resolution multiphoton imaging with a number of other imaging modalities to provide additional diagnostic values.
Clinical value and future directions
Because nonlinear microscopy can produce real-time, high-resolution images of unfixed, unstained tissues, it has potential for important applications: diagnosing and staging potentially neoplastic lesions, providing guidance for more accurate biopsy sampling, and assessing margins following tumor resection. Advances in the last several years have laid a solid foundation to overcome the technological challenges of translating nonlinear microscopy to the clinical environment.
Beyond improving on optical capabilities of the devices, however, it is critically important to verify that the excitation power used to obtain diagnostically useful images is not harmful to the tissue. While some studies have suggested that the excitation powers used for our studies are below the damage threshold,23,24 further studies of tissue damage under femtosecond pulse illumination are necessary to provide clear guidelines for the safety of MPE. These studies will undoubtedly play a major role in the design and fabrication of the next generation of multiphoton endoscopes.
ACKNOWLEDGEMENTS
We thank members of the Xu, Webb, and Weiss research groups, as well as Douglas Scherr, Sushmita Mukherjee, Ashutosh Tewari, and Manu Jain of Weill Cornell Medical College and Julie Bentley of the University of Rochester for discussions and technical suggestions. We also thank Wendy Williams of the Cornell Center for Animal Resources and Education for her assistance with the in vivo imaging experiments. Our project was supported by the National Institutes of Health (NIH)/National Cancer Institute grant R01-CA133148 and the NIH/National Institute of Biomedical Imaging and Bioengineering grant R01-EB006736.
References
1. W. Denk, J. H. Strickler, and W. W. Webb, Science, 248, 73–76 (1990).
2. I. Pavlova et al., J. Biomed. Opt., 17, 036014 (2012).
3. M. Jain et al., Arch. Pathol. Lab. Med., 136, 517–526 (2012).
4. R. Yadav et al., J. Endourol., 23, 861–867 (2009).
5. L. Fu, A. Jain, C. Cranfield, H. Xie, and M. Gu, J. Biomed. Opt., 12, 040501 (2007).
6. Y. Wu, Y. Leng, J. Xi, and X. Li, Opt. Exp., 17, 7907–7915 (2009).
7. S. Tang et al., J. Biomed. Opt., 14, 034005 (2013).
8. C. J. Engelbrecht, R. S. Johnston, E. J. Seibel, and F. Helmchen, Opt. Exp., 16, 5556–5564 (2008).
9. J. C. Jung and M. J. Schnitzer, Opt. Lett., 28, 902–904 (2003).
10. D. R. Rivera et al., Proc. Nat. Acad. Sci., 108, 17598–17603 (2011).
11. C. M. Brown et al., J. Biomed. Opt., 17, 040505 (2012).
12. D. R. Rivera, C. M. Brown, D. G. Ouzounov, W. W. Webb, and C. Xu, Opt. Lett., 37, 1349–1351 (2012).
13. D. R. Rivera, C. M. Brown, D. G. Ouzounov, W. W. Webb, and C. Xu, Opt. Lett., 37, 881–883 (2012).
14. D. M. Huland et al., Biomed. Opt. Exp., 3, 1077–1085 (2012).
15. D. M. Huland et al., Biomed. Opt. Exp., 4, 652–658 (2013).
16. Y. Fu, H. Wang, R. Shi, and J.-X. Cheng, Opt. Exp., 14, 3942–3951 (2006).
17. I. Chen, S. Chu, C. Sun, P. Cheng, and B. Lin, Opt. Quantum Electron., 34, 1251–1266 (2002).
18. N. G. Horton et al., Nat. Photon., 7, 205–209 (2013).
19. D. Kobat et al., Opt. Exp., 17, 13354–13364 (2009).
20. D. Kobat, N. G. Horton, and C. Xu, J. Biomed. Opt., 16, 106014 (2011).
21. D. G. Ouzounov, D. R. Rivera, W. W. Webb, J. Bentley, and C. Xu, Opt. Lett., 38, 3103 (2013).
22. D. G. Ouzounov et al., Biomed. Opt. Exp., 4, 1494 (2013).
23. J. M. Dela Cruz, J. D. McMullen, R. M. Williams, and W. R. Zipfel, Biomed. Opt. Exp., 1, 1320–1330 (2010).
24. R. Ramasamy et al., J. Urol., 186, 2487–2492 (2011).
David M. Huland is a PhD candidate, David R. Rivera is a postdoctoral researcher, Dimitre G. Ouzounov is a senior research associate, and Chris Xu is an associate professor in the School of Applied and Engineering Physics at Cornell University, Ithaca, NY, http://xu.research.engineering.cornell.edu. Contact Huland at [email protected].