OPTICAL DIAGNOSTICS/ONCOLOGY: Practical, sensitive and specific: Applying OCT for oral cancer screening
JENNIFER HOLTZMAN and PETRA WILDER-SMITH
Survival from oral cancer depends on the stage of the disease when first diagnosed and treated. The most common diagnostic method used, visual-tactile screening, misses early and potentially treatable cancers and has had little impact on reducing mortality rates. The method is neither highly sensitive nor highly specific, and has resulted in both false positives and false negatives. A test that misses the opportunity to optimally manage the disease invalidates effective early screening. Moreover, false negative results act as a direct disincentive to seek screening in the future.
To improve outcomes, we urgently need a screening tool with good sensitivity and specificity that will correctly identify early stages of disease, while avoiding unnecessary allocation of valuable time and resources on a false positive diagnosis.1,2
While the visual-tactile exam is non-invasive and inexpensive (requiring little more than light and a square of cotton for the examiner), it offers low sensitivity and low specificity. Caregivers apply Toluidine Blue (tolonium chloride, or TB) on suspicious areas found during visual-tactile screenings in order to improve reliability. Still, though, reliability—and thus usefulness—varies greatly depending on the exact nature of the pathology and the experience of the clinician using the stain.3
A wide range of approaches are currently being studied for their effectiveness in detecting and diagnosing oral pre-cancerous and cancerous changes.
1. Oral CDx (cytology) uses a hard brush to retrieve suspicious cells for histological examination. Though this technology offers the advantage of taking samples in a dentist's office, the high number of false positives is a major drawback.4
2. Chemiluminscent "chemi-light sticks" (Microlux DL, Vizilite) take advantage of the increased nuclear density and metabolic activity of pre-cancerous cells. Under illumination from the chemiluminescent stick, potentially premalignant or malignant lesions may appear more pale than healthy tissues. Sensitivity and specificity of this adjunct tool are still under investigation.5
3. Spectroscopy takes advantage of the altered interaction of tissue components such as natural fluorophores with light (such as PS2-OralR) when pre-cancerous or cancerous changes occur. In preliminary studies, this method has demonstrated high sensitivity—few cases of cancer will be missed. However, there is the potential for considerable number of false positives, depending on the exact approach that is used. This particular technology continues to improve as researchers address its challenges, including low signal-to-noise ratio, challenges in identifying the precise source of signals, data quantification and establishing definitive diagnostic endpoints.5
4. Photodynamics. A photodynamic approach potentially combines screening and treatment.6,7 Photoactive dyes are injected systemically or applied locally to the tissue in question, causing selective tissue fluorescence when exposed to specific wavelengths of light. Using exponentially greater light doses, the photosensitized tissues can then be used to selectively destroy the pathological tissues with minimal damage to adjacent healthy tissue. In early clinical trials, the sensitivity and specificity of this technique was determined to be very good (approx. 95%).8 The drawback is that many photosensitizers are still awaiting approval by the U.S. Food and Drug Administration (FDA).
5. Optical coherence tomography (OCT) is potentially an excellent diagnostic tool to detect and diagnose pre-malignancy and malignancy in the oral cavity. During the past decade, OCT has become something of a standard in ophthalmology; however, using OCT as an oral cancer screening tool is quite innovative.
Applying OCT for oral cancer screening
OCT uses non-ionizing light, which for oral cancer screening is applied through a hand-held probe (see Fig. 1). It produces imagery by analyzing the interference of recombined laser light waves. Preliminary studies in animals and humans with oral dysplasia and malignancy determined sensitivities between 0.83-0.93 and specificities of 0.93-0.98.9,10
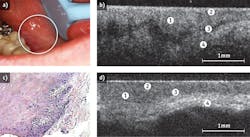
OCT images subepithelial structures that are not accessible by visual inspection, informing on changes in tissue surface and sub-surface macro- and micro-structure at levels of resolution as high as 5-10 μm, as well as identifying margins of pathological change.11,12 The technique is able to detect minute changes in the outermost 2-3 mm tissue, including the tissue architecture, mineralization, vascularity, angiogenesis and perfusion, at near-histopathological resolution (1-10 μm; see Fig. 2). The two-dimensional images that OCT generates can easily be optically stacked to produce 3D representations of the target tissues. This offers the ability to detect multiple lesions, and provides key information on potential regions of interest as well as mapping lesion extent and boundaries.In animal studies, diagnostic sensitivity and specificity for oral pathology were dramatically improved when OCT and in-vivo microscopy were combined. In pre-malignant tissues, very early changes in tissue collagen with the loss of birefringence were observed. Using OCT, the sensitivity for detecting these changes was 80%.13 Using in-vivo microscopy, the sensitivity to malignant changes improved to 87%.13 However, when OCT and in-vivo microscopy were combined, sensitivity to malignant change increased to 91%.13
Our prototype OCT oral cancer screening system is well-suited to clinical use because the miniature, hand-held probe requires just one second to accomplish both A-line scanning of up to 20 KHz and generation of a 3D data set for an area of 140 × 140 pixels (2.8 × 2.8 mm). Resolution is 5-10 μm over the entire depth of the image.
The in-vivo microscopy system we used is a Carl Zeiss META LSM510 NLO multiphoton (MPM) system. MPM is fluorescence microscopy—based on multiphoton excited fluorescence (MPF) and/or second harmonic generation (SHG)—that uses a femto-second pulsed NIR laser source. This source causes much less thermal loading of imaged tissues and increases depth of imaging comparing to "one-photon" confocal microscopy, which is important for in-vivo applications, because it permits prolonged imaging at greater tissue depths without thermally stressing the target tissues In addition, MPM permits non-invasive 3-D microscopy at a wide range of wavelengths of excitation and detection, so that surface and subsurface structures with specific fluorescence, such as collagen, or fluorescence-tagged targets, such as cells, can be imaged, and tracked throughout the body at low, medium and high-resolution.
The 91% sensitivity rating resulted from having "blinded" scorers make diagnoses based on matched sets of OCT and microscopy images.
It is interesting to note that topical application of gold nanoparticles could further improve OCT's ability to image biological tissues, by providing a 150% increase in contrast.14
Practical issues
OCT scanning is easy for clinicians to learn, and since it is non-invasive, it is relatively easy for patients to tolerate.
The approach's high specificity and sensitivity can detect early cancer lesions when they are the most treatable, yet the least detectable by more commonly used means. OCT can guide treatment to ensure that the excisions include all the dysplastic tissue and yet minimize unnecessary excision of healthy tissue.
As OCT becomes more widely incorporated into clinical practice, it is likely that its high cost will be reduced (a single unit today runs approximately $60,000). And as researchers continue to investigate the clinical utility of this imaging technique, we can expect that molecular-level information, such as those that would be produced by visible and contrast-enhancing markers, will become available.
REFERENCES
1. M.W. Lingen et al., Oral Oncol. 44: 10-22 (2008).
2. I. Gómez et al., Oral Diseases 16: 333-342 (2010)
3. W.W.Y. Su et al., JDR (2010); doi:10.1177/0022034510373763
4. V. Bhoopathi et al., Cancer 115: 1036-40 (2009)
5. M.T. Tsai et al., Optics Express 16 (20):15847-6. (2008)
6. O. Kujan et al., Cochrane Database of Systematic Reviews 3: CD004150 (2006)
7. J.S. Lestón and P. Diz-Dios, Oral Oncol. 46: 418-422 (2010)
8. M.A. Biel, Photochem. Photobiol. 83 (5):1063-8 (2007)
9. C.G. Schweitzer and M.L. Somers, Lasers Surg. Med. 42 (1): 1-8 (2010)
10. C-J Chang and P. Wilder-Smith, Plast. Reconstr. Surg. 115:1877 (2005)
11. C.S. Kim et al., J. Biomed. Opt. 14 (3): 034008 (2009)
12. J. Ridgway et al., Arch. Otolaryngol. Head Neck Surg. 132 (10): 1074-81 (2006)
13. P. Wilder-Smith et al., Lasers Surg. Med. 41 (5): 353-357 (2009)
14. C.S. Kim et al., Biomedical Optics Express 1: 106-113 (2010)
Jennifer Holtzman DDS, MPH, is Assistant Researcher, and Petra Wilder-Smith DDS, Ph.D., is Dental Director, at Beckman Laser Institute, University of California, Irvine, www.bli.uci.edu. Contact Dr. Holtzman at [email protected].