DERMATOLOGY/PHOTODYNAMIC THERAPY: The benefits of topical photodynamic therapy in dermatology
SALLY H. IBBOTSON
Tissue-localized photosensitizer, activated by light in the presence of oxygen, initiates oxidative stress, inflammation and cell death. This process, when applied clinically to treat disease, is known as photodynamic therapy (PDT), and its topical application has become increasingly popular in recent years. In dermatology, the use of topical PDT is generally used in patients with superficial, non-melanoma skin cancer or pre-cancerous skin lesions.
The two main prodrugs used for topical PDT are 5-aminolaevulinic acid (ALA) and its methyl ester (MAL). Both are precursors of the endogenous photosensitizer protoporphyrin IX (PpIX), which is expressed in all nucleated mammalian cells, but at a level low enough not to cause photosensitivity. After application to a skin tumor or lesion, both ALA and MAL are taken up into abnormal cells, and are converted to PpIX. The result is accumulation of PpIX in abnormal cells, with relatively less accumulation in surrounding normal skin. Although PDT is principally used for skin cancers, tissue-localized fluorescence can also be seen in viral warts, psoriatic plaques and other benign proliferative or inflammatory conditions after ALA or MAL application.
PpIX has peak excitation in the blue-violet light part of the spectrum (Soret band), with maximum at 410 nm. However, these wavelengths are only able to penetrate tissue to approximately 1 mm, whereas red light (with a peak waveband of 630-635 nm) penetrates up to 6 mm. For this reason, and because there is a red peak in the absorption spectrum of PpIX (albeit much smaller than for blue-violet), red light is typically used for topical PDT.
Both non-coherent and laser sources are effective for PDT, and there seems to be no significant difference in efficacy between the two. Historically, filtered xenon sources or slide projectors were used, and later, a range of fluorescent, metal halide and tungsten filament sources. Then came the development of inexpensive LED arrays, which are now the mainstay sources for topical PDT—and are enabling exciting progress in the development of ambulatory PDT. Other polymer, intense pulsed light and variable pulsed light sources can also be used, and studies have shown that even natural daylight is useful for topical PDT. Laser light sources are an alternative (although not warranted for purchase specifically for topical PDT), and typically, diode and dye lasers are used for PDT.
At the University of Dundee's Photobiology Unit (Dundee, Scotland), we use the Aktilite 16 and 128 LED sources for most treatments, although we also have a 630 nm diode laser (Diomed), a metal halide source (Waldmann 1200), a tungsten filament source (Photocure) and an additional LED source (Omnilux) (see Fig. 1). When very careful targeting of light delivery is required; for instance, at periocular sites, we may choose the 630 nm diode laser with fiber-optic light delivery. We are also increasingly exploring the use of small compact disposable LEDs for ambulatory PDT (see Fig. 2).Indications and results
The main dermatology indications for topical PDT are superficial non-melanoma skin cancer and pre-cancerous changes, known as dysplasia-notably superficial basal carcinoma (BCC), Bowen's disease (BD) and actinic keratosis (AK). For each of these, the role of PDT has been examined in randomized, controlled and open studies.1,2
Not all tumors respond well to PDT: the treatment is inferior to surgery for nodular BCCs, although it is an option when surgery is not appropriate (and, in this case, tumor debulking should be administered prior to PDT). Very heavily pigmented tumors do not respond well because melanin absorbs red light. Likewise, morphoeic, infiltrative BCCs do not selectively accumulate PpIX following ALA or MAL application, and thus do not respond well to topical PDT. Furthermore, while PDT occasionally produces initially encouraging results when applied to squamous cell carcinoma, it is associated with unacceptable recurrence rates—and because both squamous cell carcinoma and melanoma have metastatic potential, PDT is not indicated for such cases. Studies have also shown that subcutaneous metastases; for example, of breast carcinoma, do not respond well to topical PDT, likely because of poor penetration of both photosensitizer prodrug and light.
In cases where topical PDT is considered appropriate, however, it can be highly effective—at least as effective as therapies such as topical 5-fluorouracil (5-FU) and cryotherapy, and with fewer adverse effects. It is non-invasive and selective, and because it is relatively sparing of normal tissue, it can produce excellent cosmetic results. Further, it is generally well tolerated (in fact, patients tend to prefer PDT over other therapies) and treatment can be repeated on an outpatient basis.
PDT offers some other advantages, too—for instance, allowing successful treatment of large and multiple areas of BD (such as on the lower legs, and other atypical sites). It may also be of use in treating organ transplant recipients who are at markedly increased risk of developing dysplastic skin changes and non-melanoma skin cancers. The use of topical PDT in organ transplant recipients has been shown to have initial response rates for AK and BD equivalent to those in immunocompetent subjects, but with longer-term, higher relapse rates. Topical PDT may potentially delay the development of AK, but possibly not of invasive squamous cell carcinoma. In one study within subjects, topical PDT was shown to be more effective than 5-FU in treating dysplastic skin changes in immunosuppressed subjects . Interestingly, in the studies published to date, there has been no evidence of increased phototoxicity or adverse effects in immunosuppressed patients.
Carrying out topical PDT
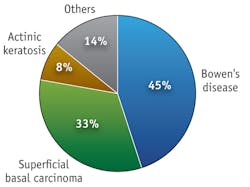
At the Photobiology Unit, University of Dundee, which is part of the Scottish Photodynamic Therapy Centre, we have used topical PDT since 1996. We have treated 1,600 patients with approximately 6,500 treatments (see Fig. 3). Most patients have either superficial BCC or BD, and about 10% are treated for AK. We also explore application in a range of other diseases. Because of PDT's selectivity of treatment, improvement in chances of healing and cosmetic outcome, we consider PDT to be a treatment of choice for field-change dysplasia, BD and superficial BCC on the lower legs, and for the treatment of multiple and large lesions at other body sites.Once we assess a patient and establish a histological diagnosis for the index lesion (or a representative lesion if multiple areas are to be treated), we map out and photograph the affected area. Metvix PDT is the licensed treatment regime for AK, BD and BCC: we administer a single treatment for the former, and two treatments one week apart for the latter two conditions.
We prepare the lesion by surface abrasion or gentle curettage without local anesthetic. For licensed indications, MAL (16%, Galderma) is applied and occluded under Tegaderm for three hours. When ALA (20%, Manmed) is used, it is applied for four hours for AK or BD, and six hours for superficial BCC.
We perform irradiation at a dose of either 37 or 75 J/cm2 using LED sources for MAL and ALA (the dose of 37 J/cm2 is included in the licensed MAL PDT regime). If a non-LED source is used, we increase the dose to 125 J/cm2, ensuring that the irradiance is less than 150 mW/cm2 to prevent a hyperthermic effect.
Resource requirements are relatively simple, and consist of the prodrug, a disposable curette or tongue depressor for surface preparation; a transparent dressing such as Tegaderm to occlude; and a UV opaque dressing such as Mepore. Once the prodrug is removed and the surface wiped, a Wood's light (UV diagnostic tool) is used for examining tumor fluorescence and for assisting in demarcation of the irradiation field—which includes a 5 mm rim of tissue that appears clinically normal. Irradiation is undertaken using a cooling fan or, if the patient finds treatment too uncomfortable, Cynosure's forced air cooling device. Immediately after treatment, redness and swelling are evident, but the main acute adverse effect is pain. Longer-term pigmentary change, scarring and ulceration are uncommon.
After three months, we assess the area and perform a second treatment cycle if necessary. Then, we follow patients for up to one year after clearance. Cosmetic outcome is generally very good and compares favorably to therapies such as radiotherapy, cryotherapy or curettage.
The pain factor
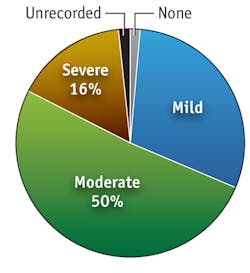
The pain of PDT can be significant and most patients experience discomfort. About 16% of patients report severe pain; another 50% describe moderate pain (see Fig. 4). Unfortunately, the pain is unpredictable and the mechanism unknown. There appears to be a neurogenic component and ALA appears to be implicated in the GABA transporter pathway. There additionally seems to be a later inflammatory component, and pain is certainly worse in the initial phase of irradiation.Certain factors apparently determine how much pain is experienced. There seems to be no difference between laser and broadband light sources in terms of pain effect, and LED and other incoherent light sources similarly appear equivalent in this regard. A recent study showed that variable pulsed light (VPL) was less painful than LED PDT. Emission spectrum is another influence: Green light causes less pain than red light for AK, although this has not been seen for BD. The total light dose delivered may not be important—perhaps because most pain is experienced in the early part of the irradiation process. However, a growing body of evidence suggests that delivery of light during PDT at lower irradiance is associated with less pain. In our own experience, irradiance of less than 50 mW/cm2 is associated with a lower proportion of patients reporting severe pain. Certainly higher irradiance devices, including LED, laser and non-coherent, seem to be associated with more pain than lower irradiance devices, whether filtered xenon, polymer, LED devices or daylight are used.
Ongoing work between the University of St. Andrews and the Photobiology Unit in Dundee has examined the use of portable low irradiance LED devices for ambulatory home PDT—which have shown very low pain scores compared with conventional PDT. This work has resulted in the development of ambulatory devices, including a "skin cancer plaster" by Ambicare Health Ltd. (which earned a CE mark in October 2009), as well as a face mask for treatment of skin cancers and acne and possibly for photorejuvenation.
Above and beyond
Topical PDT has also been applied to a diverse range of other diseases; the most studied being recalcitrant viral warts, acne, cutaneous T-cell lymphoma and psoriasis. In our experience, we have seen good/partial responses with warts, acne, T-cell lymphoma, oral lichen planus and oral leukoplakia, but poor/no response in psoriasis, melanoma metastases, porokeratosis, breast metastases, keloid, angiosarcoma or Paget's disease of the vulva.
In a recent review of topical PDT for treatment of acne, the therapy was apparently superior to no treatment, although in one study PDT was not superior to red light alone, so this requires further investigation. Repeat PDT treatments improve responses and there should be an expectation of aiming to get approximately 66% reduction in inflammatory acne, although comedonal acne is not significantly improved. Furthermore, treatment regimes currently result in prominent, phototoxic adverse effects, and patients may need to take up to a week off work after treatment.
For the future, treatment of conditions other than superficial BCC, BD and AK need further validation of use. Overall, treatment outcomes are not as optimal as they could be, and ongoing studies seek to improve both prodrug and light delivery. As an alternative to Woods' light, fluorescence spectroscopy and CCD imaging are being studied for more detailed analysis to assist, for example, in conjunction with Mohs surgery. Beyond efficacy, though, a key area of development necessary is that of pain reduction. While topical PDT offers many advantages already over other therapies, the ability to provide better patient comfort will result in much broader acceptance.
REFERENCES
1. C. Morton et al., Arch Dermatol. 142 (6): 729-35 (2006)
2. C.A. Morton et al., Br. J. Dermatol. 146 (4): 552-67 (2002)
ACKNOWLEDGEMENT
This article is based on extensively referenced paper: Photodiagnosis Photodyn. Ther. 7 (1): 16-23 (2010).
Sally H. Ibbotson, MBChB, MD, is Clinical Senior Lecturer in the Photobiology Unit, University of Dundee, Ninewells Hospital & Medical School, Dundee, Scotland, www.dundee.ac.uk/dermatology/photo/sitphot.htm, [email protected].