CELL BIOLOGY/BIOMEDICAL IMAGING: Ultraviolet becomes accessible
A trend in the development of biomedical instruments over the last decade has been to find alternatives to the gas lasers that traditionally provided excitation. Ar-ion, Kr-ion, HeNe, and other such lasers offer a huge advantage: They provide a great number of lasing lines, from UV to red, perfectly suited for optimal excitation of most of the thousands of fluorescent probes being used in biomedical applications. However, gas lasers suffer from significant drawbacks (including limited lifetime, low power efficiency, and bulky size), that have hampered evolution of the instrumentation from research to clinical environments.
Solid-state laser technologies have provided a compact, efficient, and reliable alternative, and combined with advances in instrument design and detection efficiency, and the continuous development of new fluorophores, they have opened up many new application areas for laser induced fluorescence (LIF) in both labs and clinics.
To be suitable for such uses, a laser source typically needs to operate in CW or quasi-CW mode (>10 MHz repetition rate), and provide a high-quality TEM00 beam with low-intensity noise and a high level of long-term stability. Moreover, it needs to be insensitive to temperature fluctuations, humidity, and vibrations, and have an expected lifetime of >10,000 hr. Although compact solid-state laser alternatives meeting these requirements are now available at wavelengths covering most of the visible spectrum, there has been a lack of suitable sources in the UV part of the spectrum.
The importance of being UV
Lasers in the UV are attractive for excitation of fluorophores such as Hoechst Blue, DAPI, and Indo-1. These fluorophores are growing in popularity for use in DNA analysis and calcium imaging applications, for instance in stem cell, cancer, neuroscience, and diabetes research. Their absorption maxima, in the 350–360-nm region, are nicely excited by the argon-ion laser, which offers multiple line emission in this spectral range and 100-mW output power. Frequency-tripled modelocked Nd:YAG lasers (by JDSU, for example), and more recently, frequency-tripled optically pumped semiconductor (OPS) lasers (Coherent) can offer up to several hundred milliwatts of quasi-CW or CW output power at 355 nm, and have been able to successfully provide a more compact and reliable alternative to the argon-ion laser in many applications. However, these lasers are still relatively large, high-power devices requiring substantial active cooling, and are, therefore, not optimal for integration into compact bench-top instruments. Diode lasers at 405 nm or 375 nm can provide a very compact and efficient alternative both in terms of power consumption and cost, and are frequently used for excitation of UV fluorophores. However, those wavelengths do not provide optimum excitation of many UV fluorophores, and their imperfect beam quality and poor wavelength accuracy are limiting factors in some applications.
Thus, a true CW, compact diode-pumped solid-state laser (DPSSL) in the 350–360-nm region would be an interesting alternative to the currently available UV or near-UV sources, especially when considering adding UV excitation capability to bench-top instruments targeting clinical applications. CW DPSSLs have become accepted as standard excitation sources in LIF bioanalytical tools such as confocal microscopes, flow cytometers, DNA sequencers, and high content analysis (HCA) equipment. Their high brightness, combined with sophisticated detection schemes and sensitive photon detectors, allows detection of very weak signals at very high resolution. The signals can be used for imaging of specific elements or for obtaining quantitative data on large populations.
The main challenge with attaining CW UV performance from a compact DPSSL stems from the difficulty involved in reaching required field intensities to convert photons into the UV range using nonlinear optical processes with a reasonable efficiency. But thanks to a combination of new nonlinear optical technology and innovative DPSSL designs, that challenge has been overcome (see Fig. 1).1
Insulin kinetics and other insights
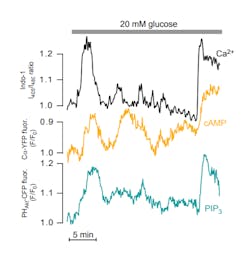
The recent introduction of compact CW 355-nm lasers has opened up novel possibilities to study intracellular signaling. One of the most significant advantages is that a UV laser light source enables improved measurements of the cytoplasmic concentration of calcium ions (Ca2+) in various types of cells. Ca2+ is a key regulator of numerous functions, including neural signal transmission, muscle contraction, and hormone secretion.
In pancreatic ß-cells, Ca2+ controls the release of insulin. Insufficient insulin secretion is the major cause of diabetes, which unfortunately is a growing disease in the richer Western world. The World Health Organization (WHO) estimates that more than 180 million people worldwide suffer from the disease and that this number will double around year 2030. Measurements of Ca2+ provide important information about insulin secretion kinetics.2
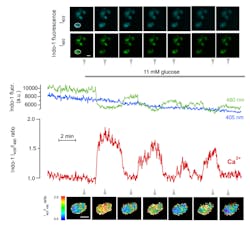
While it has been possible to monitor Ca2+ globally in the cytoplasm of individual cells for more than 25 years using epifluorescence microscopy and UV-excitable fluorescent indicators; it is often desirable to study local Ca2+ signaling in restricted sub-cellular compartments. One such compartment is the cytoplasm beneath the plasma membrane, where much of the important signaling and the actual release of insulin secretory granules occur. The sub-membrane space can be selectively visualized with total internal reflection fluorescence (TIRF) microscopy, which uses an evanescent wave created by the reflection of a laser beam at the interface between a glass coverslip and the aqueous cytoplasm of adherent cells. TIRF measurements of Ca2+ have typically relied on indicators with visible wavelength absorption and single wavelength excitation and emission, with the drawback that the recorded signal is sensitive to uneven dye loading, photobleaching, dye leakage, and focus changes. Excitation with a CW 355-nm laser now allows TIRF imaging with the Ca2+ indicator Indo-1, whose emission spectrum shifts upon Ca2+ binding, thereby allowing ratiometric Ca2+ recordings (see Fig. 2). The use of a UV-excitable indicator provides the additional advantage that Ca2+ recordings can more easily be calibrated and they can be performed without spectral interference from fluorescent proteins; other parameters can therefore be simultaneously investigated.Uncaging compounds and more
Another important application is uncaging of photoprotected compounds or "caged compounds." In this technique the concentration of ions, signaling molecules, or other biologically active compounds, can be instantaneously changed with a flash of UV light. The role of a particular signaling step can thereby be investigated independent of the preceding steps in the signal transduction chain. UV flashlamp devices have been commercially available for a long time but are bulky and show poor beam parameters compared to a laser source. A compact laser is thus superior when adapting uncaging technology to custom-built TIRF and other microscopy setups. Frequency-tripled pulsed Nd:YAG lasers are an alternative but usually require heavy attenuation to avoid disturbing the experiment with the high-energy pulses.
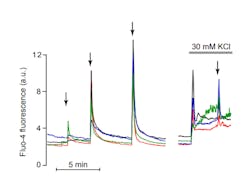
In an experiment involving uncaging of Ca2+ in insulin-secreting cells loaded with NP-EGTA, the UV laser-induced change in sub-membrane cytoplasmic Ca2+ is monitored by TIRF imaging of the visible wavelength indicator Fluo-4 (see Fig. 4). However, other probes can also be used for parallel imaging of other messengers or signaling proteins.Given the importance of Ca2+, the availability of affordable and compact UV sources has implications for research in many other areas, such as neurological disorders, cancer, and heart disease.
And certainly the use of compact CW 355-nm lasers is not restricted to Ca2+ measurements, either. Because several other popular fluorescent probes with broad application in cell biology have UV absorption, they are also applicable to flow cytometry. A new generation of user-friendly, bench-top flow cytometers capable of parallel multiparameter analysis is being enabled by the development of new laser technology–and compact UV lasers extend the palette of fluorophores available to them.
The authors would like to thank Oleg Dyachok and Olof Idevall-Hagren of Uppsala University for their contributions to this article.
References
- J. Hellström et al., 7578–26, Photonics West 2010.
- A. Tengholm and E. Gylfe, Mol Cell Endocrinol., 297:58–72, 2009.
- O. Dyachok et al., Nature, 439:349–52, 2006.
- O. Idevall-Hagren and A. Tengholm, J. Biol. Chem., 281:39121–7, 2006.
- O. Dyachok et al., Cell Metab., 8:26–37, 2008.
About the Author
Elizabeth Illy
Director of Marketing, Cobolt (HÜBNER Photonics)
Elizabeth Illy is director of marketing at Cobolt, a part of HÜBNER Photonics (Solna, Sweden).
Anders Tengholm
Researcher, Department of Medical Cell Biology at Uppsala University
Anders Tengholm is a researcher at the Department of Medical Cell Biology at Uppsala University (Uppsala, Sweden).