MULTIPHOTON MICROSCOPY/SUPER-RESOLUTION IMAGING: Licensing deals, developments bring new microscopes to market
What's new in super-resolution and multiphoton microscopy? Plenty. A number of announcements from commercial developers emerged from two recent events, the American Society for Cell Biology (ASCB) Annual Meeting (December 5–9, 2009; San Diego, CA) and Neuroscience 2009 (the Society for Neuroscience's annual meeting, October 17–21, 2009; Chicago, IL). That's no surprise, because these techniques offer tremendous advantages for neuroscientists and cell biologists whose demanding microscopy requirements include the need to see subcellular structures and to image in real time.
At ASCB, Nikon Instruments (Melville, NY) announced both super-resolution and multiphoton technologies. For instance, the company has added multiphoton excitation capability for its A1R confocal system, which offers frame rates from 30 to 420 fps; new optics promise deeper imaging with wider spectral corrections and the highest possible numerical apertures in water and oil immersion formats.
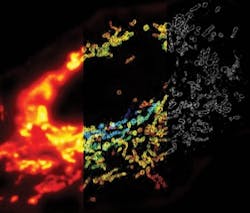
In addition, Nikon announced two licensing deals–an exclusive one with Harvard University for Stochastic Optical Reconstruction Microscopy (STORM), a technology that exceeds traditional diffraction limits by an order of magnitude and enables 3D image acquisition (see Fig. 1). Nikon's N-STORM system, planned for release in May, promises resolutions higher than ever before achieved by conventional optical microscopes: lateral resolution to ~20nm and axial to ~50nm. It will be based on Nikon's Eclipse Ti research inverted microscope.
The STORM technique uses photo-switchable fluorescent probes to temporally separate the otherwise spatially overlapping images of individual molecules. It reconstructs fluorescence images (2D and 3D) using fluorophore localization information calculated from multiple exposures, generating substantial information from detection of single molecule fluorescence emissions to enable both structural and molecular understanding of specimens.
Similarly, Nikon has licensed Structured Illumination Microscopy (SIM) technology from the University of California, San Francisco for production of the Super Resolution Microscope N-SIM, also due out in May. Like N-STORM, N-SIM is being designed upon Nikon's Eclipse Ti research inverted microscope–with CFI Apo TIRF 100x oil objective lens (N.A. 1.49).
The SIM technique can produce twice the resolution–in all three directions–of conventional commercial optical microscopes. It also promises the fastest imaging capability in the industry, with a time resolution of 0.6 sec/frame. The technology combines a special illumination pattern with moiré fringing (which reveals information not resolvable by down-modulating high frequencies) and computational image analysis. The newly developed TIRF-SIM illumination approach enables Total Internal Reflection Fluorescence (TIRF) observation with higher resolution than conventional TIRF microscopes for detailed structural information near the cell membrane. Yet another new 3D-SIM technique enables optical sectioning of specimens.
Another approach to SIM
During the summer, though, Carl Zeiss MicroImaging GmbH (Jena, Germany) also announced an agreement with UCSF for commercialization of SIM and the right to integrate the technique into Zeiss's microscope systems–and at Neuroscience 2009, the company announced a new product family that allows SIM–and Zeiss's photoactivated localization microscopy (PAL-M) approach–to be comprehensively used and individually combined for the first time.Zeiss explains that the key benefits of PAL-M are XY resolution to 20 nm, depending on sample, Z-resolution to ~100 nm (thanks to a new 1.57 NA TIRF lens), and the ability to collect two imagery channels–plus easy-to-use software. And key benefits of SR-SIM include the ability to collect up to four channels; to work with standard dyes, fixatives, and conventional high-quality coverslips; plus acquisition speeds of ~2 fps and resolution.
Zeiss is calling its new three-instrument product line ELYRA. The name derives from Epsilon Lyrae, which appears as a single star (in the constellation Lyra) when viewed with the unaided eye, but is clearly seen as two stars with the help of binoculars–and as two pairs of stars at stronger magnification. ELYRA S.1 (SR-SIM) was announced as the first microscope system to allow the use of SR-SIM technology on the basis of a standard microscope stand. ELYRA P.1 provides PAL-M technology commercially for the first time, while the ELYRA PS.1 embodies the first combination of the two technologies on the same stand, both with each other and with a laser scanning microscope (LSM)–enabling imaging of a specimen with the three techniques in succession.
Next step from STED
Also in October, Leica Microsystems (Wetzlar, Germany), which has had an exclusive license to commercialize another super-resolution microscopy technique (stimulated emission depletion or STED, from Max Planck Institute, Göttingen, Germany), announced an additional exclusive license from MPI for a newer method. Leica will implement an approach known as GSDIM (ground state depletion microscopy followed by individual molecule return), which enables nanometer-level resolutions even in conventional widefield microscopes. A strength of GSDIM is that it uses conventional fluorescence markers (including fluorescent proteins and rhodamines) to image proteins and other biomolecules inside cells to a few nanometers.
With GSDIM, the fluorescent molecules in the specimen are almost completely switched off using laser light. However, individual molecules spontaneously return to the fluorescent state, while their neighbors remain non-illuminating. In this way, the signals of individual molecules can be acquired sequentially using a highly sensitive camera system, and their spatial position in the specimen can be measured and stored. An extremely high-resolution image can then be created from the position of many thousands of molecules. This enables cell components that are situated very close to one another and cannot be resolved using conventional widefield fluorescence microscopy to be spatially separated and sharply imaged.
First commercial "planar" system
On display at Neuroscience were a number of exciting microscopy systems from lesser-known developers, including what seems to be the first commercial light sheet (a.k.a. planar) microscopy system. LaVision BioTec's (Bielefed, Germany) Ultramicroscope provides sub-cellular resolution for samples even a centimeter in size. Based on a technique originally developed for particle analysis in 1903 (by H. Siedentopf and Nobel laureate R. Zsigmondy), it uses a thin uni- or bi-directional sheet of light (3–40 µm thick and 0.1–10 mm wide) to excite biological samples, while a CCD perpendicular to the region of interest records the entire plane of illumination. By moving the sample through the sheet the system can generate 3D images of whole organs (such as a mouse brain), using fluorescent proteins, fluorescent dyes, or autofluorescence.
The technique represents the best aspects of dark field, confocal, and fluorescence microscopy says Sid Ragona, whose company Ragona Scientific (Pittsford, NY) distributes the system in North America. "From the dark field aspect, the incident light is at 90 degrees to the collection optics. From the confocal aspect, no confocal apertures are required since there is no illumination above or below the plane of illumination. From the fluorescence aspect, it means that there is no illumination above and below the plane of illumination (as there is in confocal microscopy) thus there is no bleaching of the sample that is not being illuminated."
For large samples, the tissue must be infused with a solution having the same refractive index as the protein. No other technique allows such rapid optical sectioning of large samples, Ragona explains.Real-time multiphoton LSM
Another Neuroscience exhibitor, Femtonics (Budapest, Hungary), demonstrated its Femto3D-RC, "the first real-time (150 Hz) 3D multiphoton laser scanning microscope on the market." An upgrade to the company's Femto2D, the new system is due out in early 2010. The company notes that conventional microscopes create 3D images by moving the objective focusing arm–and because this approach is inherently slow, they are able to resolve rapid biological processes only in the focal plane of the objective. Femto3D, on the other hand, collects signals from different depths of the sample quickly enough to resolve functionality in 3D space, and with a high signal-to-noise ratio. The company's patent-pending approach precisely synchronizes XY free line scanning (using galvanometric mirrors) with nonlinear resonance periods of the z-axis movement in the objective. The approach reportedly yields a 3D trajectory selected to match the path of the dendritic shafts and spines of neurons without limiting pixel dwell time.
About the Author

Barbara Gefvert
Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.