Correlative microscopy views cellular distribution of membrane receptors structurally
By incorporating a 5.5 Mpixel scientific CMOS (sCMOS) camera into their correlative microscopy setup, researchers at Osaka University (Japan) and colleagues have demonstrated that microvilli projections around the cell surface mediate the adhesion of circulating leukocytes to endothelium, a process that is critical for their trafficking in vasculature.
Related: A powerful pairing for cell studies: Correlative light and electron microscopy
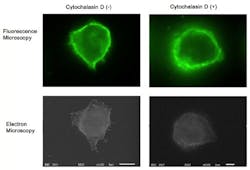
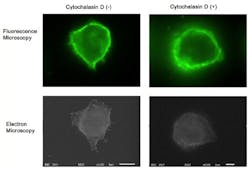
Although fluorescence imaging is a powerful approach to studying the cellular distribution of membrane receptors, it is limited in its ability to display fine structural information. Therefore, the team mapped the distribution of CD44 (a cell-surface glycoprotein) at the lymphocyte cell surface in liquid by correlative immune-fluorescence optical and immuno-electron microscopy using a combined fluorescence and atmospheric scanning electron microscope. Once CD44 cell surface labeling was confirmed with the images from the sCMOS detector, atmospheric SEM revealed the microvilli on the cell surface with the localization of label on the microvilli.
"Dual labeling was employed using an anti-CD44 monoclonal antibody and a secondary antibody conjugated with Alexa Fluor 488 and 1.4 nm positively charged Nanogold particles," says Toshiyuki Murai, who led the work. "We also demonstrated that treatment of cells with cytochalasin D resulted in the loss of the microvilli projections and the simultaneous nullification of CD44-mediated adhesion to its ligand hyaluronan. These results suggest the functional relevance of microvilli in CD44-mediated rolling adhesion under shear flow."
The correlative light and SEM imaging was accomplished by culturing the mouse lymphocytes in a petri dish to which a 100 nm silicon nitride window was fixed. This window is transparent to the electron beam, which is projected from beneath the dish by the inverted SEM, while the upright fluorescence microscope excites the sample from above through the objective lens.
Full details of the work appear in the International Journal of Molecular Sciences; for more information, please visit http://dx.doi.org/10.3390/ijms141020809.
-----
Follow us on Twitter, 'like' us on Facebook, connect with us on Google+, and join our group on LinkedIn