Human-safe injectable agent provides fluorescence guidance during cancer surgery
Doctors at the Duke University School of Medicine (Durham, NC) have tested a new injectable agent that causes cancer cells in a tumor to fluoresce, potentially increasing a surgeon's ability to locate and remove all of a cancerous tumor on the first attempt. The imaging technology was developed through collaboration with scientists at Duke, the Massachusetts Institute of Technology (MIT; Cambridge, MA), and Lumicell (Wellesley, MA).
Related: Advanced surgery: NIR fluorescence guidance arrives
A trial at Duke University Medical Center involving 15 patients undergoing surgery for soft-tissue sarcoma or breast cancer found that the injectable agent, a blue liquid called LUM015, identified cancerous tissue in human patients without adverse effects. Cancer surgeons currently rely on cross-sectional imaging such as magnetic resonance imaging (MRI) and computed tomography (CT) scans to guide them as they remove a tumor and its surrounding tissue. But in many cases, some cancerous tissue around the tumor is undetected and remains in the patient, sometimes requiring a second surgery and radiation therapy.
The trial marks the first protease-activated imaging agent for cancer that has been tested for safety in humans, according to the study's senior author David Kirsch, MD, Ph.D., a professor of radiation oncology and pharmacology and cancer biology at Duke University School of Medicine.
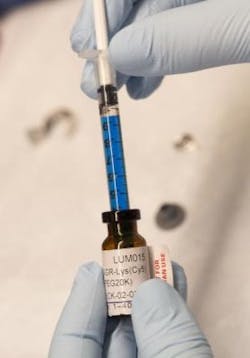
LUM015 was developed by Lumicell, a company started by researchers at MIT and involving Kirsch. In companion experiments in mice described in the study, LUM015 accumulated in tumors where it creates fluorescence in tumor tissue that is on average five times brighter than regular muscle. The resulting signals aren't visible to the naked eye and must be detected by a handheld imaging device with a sensitive camera, which Lumicell is also developing, Kirsch says.
In the operating room after a tumor is removed, surgeons would place the handheld imaging device on the cut surface. The device would alert them to areas with fluorescent cancer cells.
Going into surgery, the goal is always to remove 100% of the tumor, plus a margin of normal tissue around the edges, explains senior author Brian Brigman, MD, Ph.D., chief of orthopedic oncology at Duke. Pathologists then analyze the margins over several days and determine whether they are clear.
Brigman, who is also the director of the sarcoma program at the Duke Cancer Institute, explains that if LUM015 is successful in subsequent trials, it would significantly change sarcoma treatment. If it is possible to increase the cases where 100% of the tumor is removed, the research team could prevent subsequent operations and potentially cancer recurrence. Knowing where there is residual disease can also guide radiation therapy, or even reduce how much radiation a patient will receive, he says.
Researchers at Massachusetts General Hospital are currently evaluating the safety and efficacy of LUM015 and the Lumicell imaging device in a prospective study of 50 women with breast cancer. Afterward, Kirsch says, multiple institutions would likely evaluate whether the technology can decrease the number of patients needing subsequent operations following initial breast cancer removal.
Full details of the work appear in the journal Science Translational Medicine; for more information, please visit http://dx.doi.org/10.1126/scitranslmed.aad0293.
Follow us on Twitter, 'like' us on Facebook, connect with us on Google+, and join our group on LinkedIn