Method pushes super-resolution microscopy to distinguish individual features in single molecules
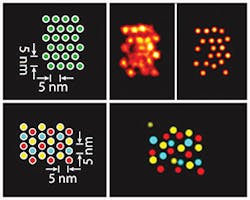
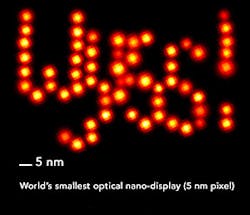
A team of researchers at Harvard University's Wyss Institute for Biologically Inspired Engineering (Boston, MA) developed a method called discrete molecular imaging (DMI) that allows its DNA nanotechnology-powered super-resolution microscopy platform to tell apart features distanced 5 nm from each other in a densely packed, single molecular structure.
Related: Startup to provide access to low-cost super-resolution microscopy
"The ultra-high resolution of DMI advances the DNA-PAINT platform one step further towards the vision of providing the ultimate view of biology. With this new power of resolution and the ability to focus on individual molecular features, DMI complements current structural biology methods like x-ray crystallography and cryo-electron microscopy. It opens up a way for researchers to study molecular conformations and heterogeneities in single multi-component complexes, and provides an easy, fast, and multiplexed method for the structural analysis of many samples in parallel," says Peng Yin, Ph.D., who is a Core Faculty member at the Wyss Institute as well as Professor of Systems Biology at Harvard Medical School (also in Boston, MA).
DNA-PAINT technologies, developed by Yin and his team, are based on the transient binding of two complementary short DNA strands, one being attached to the molecular target that the researchers aim to visualize and the other attached to a fluorescent dye. Repeated cycles of binding and unbinding create a very defined blinking behavior of the dye at the target site, which is highly programmable by the choice of DNA strands and has now been further exploited by the team's current work to achieve ultra-high resolution imaging.
The Wyss Institute scientists have benchmarked the ultra-high resolution of DMI using synthetic DNA nanostructures. Next, the researchers plan to apply the technology to actual biological complexes such as the protein complex that duplicates DNA in dividing cells or cell surface receptors binding their ligands.
Full details of the work appear in the journal Nature Nanotechnology; for more information, please visit http://dx.doi.org/10.1038/nnano.2016.95.