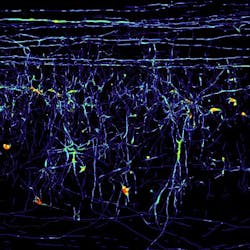
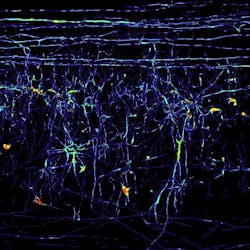
Microscopy method renders spinal cord tissue transparent
When spinal cord injury occurs, the axons (long nerve cell filaments) may become severed. Investigation on whether or not these axons can be stimulated to regenerate has been going on for a whileâgrowth that takes place on a scale of only a few millimeters. Realizing this, researchers at the Max Planck Institute for Neurobiology (Martinsried, Germany) and international colleagues have developed a new microscopy method to observe single nerve cells in intact tissue and in 3-D.
So far, researchers have only been able to observe axon growth by cutting the tissue in question into ultra-thin slices and examining them under a microscope. But the two-dimensional sections provide only an inaccurate picture of the spatial distribution and progression of the cells. In exceptional cases, researchers could go to the trouble of first digitizing each slice and then reassembling the images, one by one, to produce a virtual 3-D modelâbut the endeavor requires days and sometimes weeks to process the results of just one examination. What's more, mistakes can easily falsify the results: The appendages of individual nerve cells might get squashed during the process of slicing, and the layers might be ever so slightly misaligned when set on top of each other.
The Max Planck research team's new method is based on a technique known as ultramicroscopy, which was developed by Hans Ulrich Dodt from the Technical University of Vienna in Austria. Taking this technique a step further, the Max Planck team recognized that spinal cord tissue is opaque due to the fact that the water and the proteins contained in it refract light differently. So, they removed the water from a piece of tissue and replaced it by an emulsion that refracts light in exactly the same way as the proteins, leaving them with a completely transparent piece of tissue.
By using fluorescent dyes to stain individual nerve cells, the researchers could now trace their path from all angles in an otherwise transparent spinal cord section. This enabled them to ascertain once and for all whether or not these nerve cells recommenced their growth following injury to the spine. Frank Bradke of the Max Planck team notes that their method can also be applied to other kinds of tissue; for example, the blood capillary system or the way a tumor is embedded in tissue could be portrayed and analyzed in 3-D.
The team's work has been published in Nature Medicine; for more information, please visit http://www.nature.com/nm/journal/vaop/ncurrent/full/nm.2600.html.
-----
Follow us on Twitter, 'like' us on Facebook, and join our group on LinkedIn
Follow OptoIQ on your iPhone; download the free app here.
Subscribe now to BioOptics World magazine; it's free!