Super-resolution microscopy of large fields in living cells possible with sCMOS
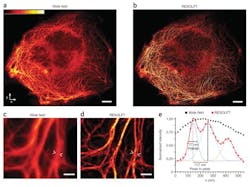
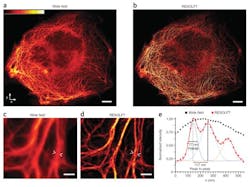
Super-resolution microscopy using a novel fluorescence microscope has enabled researchers from the Max Planck Institute (Göttingen, Germany) to perform real-time nanoscopic imaging of large fields of living cells for the first time. The microscope, which is equipped with a low-noise, high-speed 5.5 Mpixel scientific CMOS (sCMOS) camera, delivers massive parallelization techniques to create 116,000 simultaneous scanning points and super-resolve 120 × 100 µm fields in <1 s.
Related: Nobel Prize honors super-resolution optical microscopy
The research was led by 2014 Nobel Prize winner Prof. Stefan Hell, who first advanced stimulated emission depletion (STED) and a technique dubbed Reversible Saturable/Switchable Optical Fluorescence Transitions (RESOLFT), both of which are far-field, super-resolution microscopy methods. Although RESOLFT can capture images at video rates, imaging speed has been governed by the kinetics of fluorophore state transition and, more importantly, the number of scanning steps required to cover the field of view—until now.
Hell and his research team reconciled the major goals of nanoscopy development: low-intensity operation, large fields of view, and fast recording, at a resolution not limited by diffraction. They demonstrated that RESOLFT nonlinear structured illumination can be parallelized using two incoherently superimposed orthogonal standing light waves. The intensity minima of the resulting pattern act as imaging "doughnuts," providing isotropic resolution in the focal plane and making pattern rotation redundant.
Full details of the work appear in the journal Nature Methods; for more information, please visit http://dx.doi.org/10.1038/nmeth.2556.
-----
Follow us on Twitter, 'like' us on Facebook, connect with us on Google+, and join our group on LinkedIn
Subscribe now to BioOptics World magazine; it's free!