Advanced microscopy helps demystify wound healing
Funded by the Biotechnology and Biological Sciences Research Council (BBSRC), researchers from the University of Birmingham in England are using advanced microscopy techniques to uncover the early stages of wound healing to help improve our understanding of how the human body heals itself.
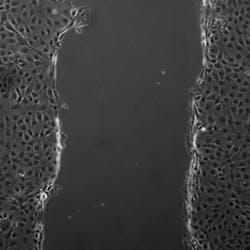
Cell migrationâthe mechanism by which cells move to new locationsâis important in a number of clinical processes, yet no one really knows how it happens, particularly when groups of cells move together such as during wound healing. Sometimes cells don't move when we would like them to (as in ulcers), while other cells migrate when we don't want them to (like when cancers spread through the body). The researchers have been investigating how a process called endocytosis (how cells take in substances from their surroundings) could play a key role in the cell migration involved in repairing damage to organs such as the skin, lungs, and kidney.
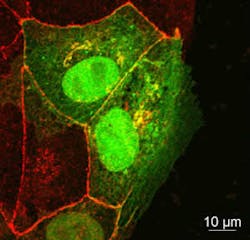
Using microscopes in the Birmingham Advanced Light Microscopy (BALM) facility, the researchers showed that migrating cells used endocytosis to take in proteins from their surface. The key, though, was to find which of these proteins was the most important.
Led by Dr. Josh Rappoport, the research team identified two candidate protein molecules that they suspected played a key role in migration. These proteins both help to moor a cell in place and act as sensors by which cells detect changes in their environment. Based on what researchers had seen in other types of cells, the team initially expected that the cells on the edge of a wound would use endocytosis to draw in the molecules that make structures called focal adhesions, which allow a cell to cling to its surroundings. However, when they used total internal reflection fluorescence (TIRF) microscopy to study migrating cells, they found that endocytosis was not involved in rearranging the focal adhesions during wound healing.
Instead, the team uncovered a different story. Using confocal microscopy, the team found that a different protein, called occludin, was being taken in via endocytosis.
In healthy tissue, occluding holds epithelial cells together to form a single flat layer. When the researchers created a layer of epithelial cells in culture and then made a wound, the cells on the edge pulled occludin in via endocytosis and then moved forward to fill the gap. But when the researchers added additional occludin to the spaces between cells, the wound was not filled as quickly.
The research has been published in Biology of the Cell; for more information, please visit http://onlinelibrary.wiley.com/doi/10.1111/boc.201100004/abstract.
-----
Follow us on Twitter, 'like' us on Facebook, and join our group on LinkedIn
Follow OptoIQ on your iPhone; download the free app here.
Subscribe now to BioOptics World magazine; it's free!