Confocal microscopy system captures large groups of neurons in living animals
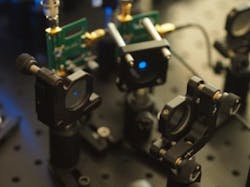
Recognizing that imaging large groups of cells requires capturing cellular or subcellular details at fast speeds over a large 3D volume, a team of researchers at Boston University (BU; Boston, MA) has developed a confocal microscopy system that can image large groups of interacting cells in their natural environments. The system could provide an unprecedented view into how large networks of neurons interact during various behaviors.
In their paper that describes the work, the researchers show that their "multi-z" confocal microscopy system can image the brains of living mice at video rate and with a field of view larger than 1 mm. "We found a way to merge the needed imaging features in a microscopy system that is easy to build and operate," says Amaury Badon, first author of the paper. "It also provides results in real time without the need for complicated data analysis or image processing."
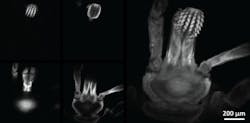
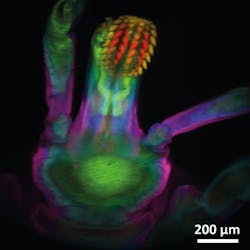
The researchers used the new microscope to image a tick. On the left are images from various depths, which were reconstructed into the volume image on the right. (Image credit: Amaury Badon, Boston University)
Confocal microscopy produces images with high resolution and contrast by using a physical pinhole to block out-of-focus light and let in-focus light through. However, scanning a sample to acquire enough 2D images to reconstruct a 3D volume is time-consuming and produces large amounts of data.
To acquire multiple planes simultaneously, the researchers developed a way to reuse the light for imaging cells in one plane to also image cells deeper in the sample. They used an approach called extended illumination in which the microscope's objective lens is only partially filled with the illuminating light, allowing the light to reach deeper into the sample. The full objective lens is then used to detect fluorescence, which provides high resolution. Rather than having one pinhole like traditional confocal setups have, the new microscope has a series of reflective pinholes that each capture in-focus light from a different depth within the sample.
The researchers built this detection unit in which light is reflected from one pinhole to the next one, allowing the microscope to probe different depths within a sample. (Image credit: Amaury Badon, Boston University)
The research team also tailored the microscope for larger-scale imaging than conventional confocal microscopes and designed it to image at video rate. Fast image acquisition was important because the fluorescence indicators that monitor cellular function typically operate on time scales of a few tens of milliseconds.
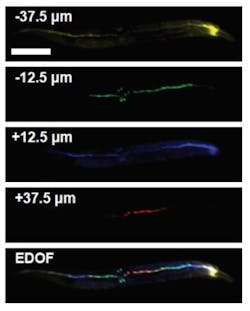
The researchers demonstrated the multi-z confocal microscopy system by using it to image whole C. elegans worms, which are too large (500–800 µm long) to easily image all at once with a traditional confocal microscope. They simultaneously detected and monitored the activity of 42 neurons in the whole organism, even when the worms were moving.
The researchers demonstrated the multi-z confocal microscopy system by using it for Simultaneous imaging of a single worm at different depths (color corresponds to depth). The bottom image shows the resulting volumetric rendering. (Image credit: Amaury Badon, Boston University)
They then used their microscope to image the hippocampal region of a mouse brain in an awake animal whose head was kept stationary. They were able to image neuron activity within a volume measuring 1200 × 1200 × 100 µm at video rate. Using an algorithm, the researchers were able to identify 926 neurons in the imaged volume.
A colorized version of the tick volume image at the top is shown. (Image credit: Amaury Badon, Boston University)
They are now working to improve the speed and depth penetration of the technique as well as to make the microscope as versatile and user-friendly as possible.
Full details of the work appear in the journal Optica.