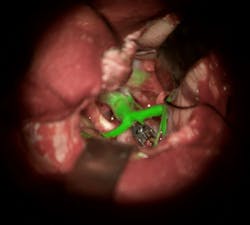
U.S. FDA clears surgical fluorescence solution for augmented reality
Leica Microsystems (Buffalo Grove, IL) has received U.S. FDA approval to market its augmented reality (AR) GLOW800 surgical fluorescence for vascular neurosurgery. In combination with the indocyanine green (ICG) fluorescent dye, GLOW800 allows surgeons to observe cerebral anatomy in natural color, augmented by real-time vascular flow in a single image, with full depth perception. This AR solution provides the surgeon a complete view of anatomy and physiology to support crucial decisions and actions during vascular neurosurgery.
GLOW800 AR fluorescence is the first of many imaging modalities that will be based on the GLOW AR platform from Leica Microsystems. GLOW AR modalities can be fully integrated in the ARveo digital AR microscope, which launched earlier in 2018. Following the U.S. FDA clearance of GLOW800, ARveo customers in the U.S. can experience the full advantages of AR visualization in the operating room.
For more information, please visit www.leica-microsystems.com.