Advanced cell imaging for sale
No longer are optical-tweezer systems limited to build-it-yourself researchers. Now available commercially, they enable real-time, high-resolution 3-D live-cell imaging without perturbation.
By Joost van Mameren
The use of nanoparticles as sensors and probes enables important developments in medicine, and in molecular and cell biology. And the ability to observe the entrance process of a particle into a cell—in real time, in 3-D, and without perturbation of the biological specimen—is a dream of many life-science researchers.
This capabability is now available through JPK Instruments’ NanoTracker optical tweezers and 3-D particle tracking system, which provides live-cell imaging at a new level. With the NanoTracker, a user can trap and track particles measuring between several microns and 30 nm, with the ability to control, manipulate, and observe vesicles, endosomes, gene and drug spheres, viruses and bacteria, nanoprobes or nanoprobe-carriers, biomarkers, or even whole cells in real time with nanometer precision. It offers a live-cell imaging technique with high temporal and spatial resolution applied to nonlabeled nanoparticles. This capability portends new applications in many disciplines, including biophysics, biochemistry, cellular and medical research in microbiology, developmental and systems biology, infection research and immune response, toxicity of nanoparticles, and many more.
Traditional approaches to observing particles—such as epifluorescence, total-internal-reflection fluorescence (TIRF), laser-scanning microscopy, and video particle tracking—have several drawbacks: labeling is time-consuming and causes perturbation in single molecule experiments. The best resolution in conventional confocal laser scanning microscopy (CLSM) is approximately 300 nm in the x and y dimensions, and approximately 500 nm in the z axis. Fluorescence is not label-free and has poor performance for processes occurring on cell membranes. TIRF measures only the first 200 nm from the surface of a cell and gives no access to the whole cell volume. Particle tracking by video microscopy is only a two-dimensional technique with best resolution of 15 nm and poor temporal resolution.
Introduction to optical tweezers
Optical tweezers technology has evolved from proof-of-principle experiments to an established quantitative technique in fields ranging from (bio)physics through cell biology. As the name suggests, optical tweezers provide a means to manipulate objects with light. With this technique, microscopically small objects can be held and manipulated. At the same time, the forces exerted on the trapped objects can be accurately measured.
The physical principle underlying optical tweezers is the radiation pressure exerted by light when colliding with matter. For macroscopic objects, the radiation pressure exerted by typical light sources is orders of magnitude too small to have any measurable effect: we do not feel the light power of the sun pushing us away. However, for objects of microscopic dimensions (<100 µm) radiation pressure can have considerable effect.
Optical tweezers, also known as optical traps, have been used extensively, not only to manipulate biomolecules and cells, but also to directly and accurately measure the minute forces (on the order of fractions of picoNewtons) involved. Most often, the biomolecules of interest are not trapped themselves directly, but manipulated through functionalized microspheres.
The correct physical description of optical trapping depends on the size of the trapped object. One speaks of “ray optics” when the object’s dimension d is much larger than the wavelength of the trapping light: d>>λ. In this case, diffraction effects can be neglected and the trapping forces of the light can be understood in terms of ray optics. The approach described by d<<λ is called the Rayleigh regime. In this case, the trapped particles can be treated as point dipoles, as the electromagnetic field is constant on the scale of the particle. Obviously, if the laser is not focused, the particle will be propelled away due to the forward radiation pressure caused by light scattering.
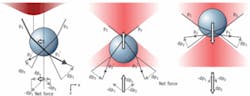
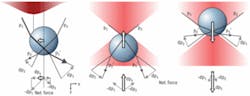
In ray optics, the origin of the trapping force can be intuitively understood in terms of refraction of light rays between media with different indices of refraction (see Fig. 1). The lateral gradient restoring force (see Fig. 1, left) can be understood as follows: if rays p1 and p2 have different intensity, the momentum changes of these rays (Δp1 and Δp2, respectively) differ in magnitude, causing a net reaction force on the refracting medium in the direction of highest intensity. The x projection of this force Δpx tends to counteract a displacement from the laser beam axis, pulling the particle to the center of beam.
The axial gradient force is similarly caused by momentum transfer upon refraction, resulting in a restoring force towards the focus (see Fig. 1, center and right). The scattering force would cause the object to be propelled out of the focus (along the positive z direction). The object is stably trapped only if the scattering force along the positive z direction is compensated by the gradient force along the negative one. To achieve this, a tight focus is needed, with a significant fraction of the incident light coming in from large angles.
Experimental platform
NanoTracker technology provides precisely quantifiable and reproducible measurements of particle/cell interactions. The system delivers precise information about single molecule mechanics and may also be used to determine mechanical characteristics such as adhesion, elasticity or stiffness.
Optical tweezers can be used in for a wide variety of applications, including single molecules and biopolymers, measuring forces, DNA/RNA, motor-proteins, sugars, molecular mechanics (pulling, stretching), cell membranes, lateral organization (e.g., lipid rafts), trans-membrane processes, single binding events, cell-particle interaction, sytotoxicity, nanotoxicity, endocytosis, phagocytosis, biological barriers, local gene or drug delivery, biral and bacterial infection, entrance mechanism studies, and tracking of pathogen-host interaction.
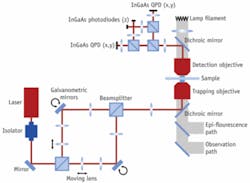
Until now, most optical-tweezer systems have been custom-built by researchers for their own needs. There are variants consisting of different laser setups, single or multiple optical traps, and different optical detection techniques. The NanoTracker offers the first system with full integration into an inverted research microscope and extensive environmental control of the sample (see Fig. 2). In general, experiments require long-term system stability—which is achieved with the choice of an ultrastable 1064 nm laser, a folded optical pathway in combination with a drift-compensated design (see Fig. 3).
Software has been designed to give operating modes including a 3-D volume scan, “classic” pulling or unfolding with two traps, particle tracking by trailing trap, local rheology and viscosity measurements, or correlated probing with a reference trap. The “Point and Trap” feature makes catching a particle almost routine.
Measuring forces with optical tweezers can be done with very high accuracy over a wide range of forces. The typical forces exerted and explored by optical tweezers range between 0.1 and 300 pN. As such, optical tweezers are complementary to other force-sensing techniques such as atomic-force microscopy, which is typically operated in a higher force regime.
The spatial resolution of optical tweezers is also impressive. Depending on the application, optical tweezers can be alternatively applied to measure displacements from the trap center, rather than exerted forces. This can be used for nanometer-resolution particle tracking, which, combined with the high bandwidth of quantitative optical tweezers, allows the real-time study of kinetic binding or motility.
Now accepting applications
Since their invention, optical tweezers have found their most prominent quantitative applications in the field of single-molecule biophysics. As such, they have been used to unravel the complex elasticity of biopolymers—DNA, RNA, proteins—for which measurements could complement those performed with other single-molecule force spectroscopy techniques such as AFM.
They have also contributed a great deal to the detailed understanding of how many “motor proteins” convert chemical energy into work. Milestone papers in the kinesin and myosin field owed their first direct mechanical insights to optical tweezers measurements.1, 2 These pagers have sparked many follow-up research efforts and a plethora of mechanically active enzymes have been studied since then, including many involved in DNA metabolism.
The number of studies in this field increases steadily, as many questions in biology and biophysiology remain open. These efforts have helped push quantitative optical tweezers technology to its current level. The power of optical-tweezers instrumentation to directly and in real time measure picoNewton forces and nanometer displacements, spot-on in the realm where many such biological motors are active, has secured a central place for this cornerstone technique.
More recently, optical tweezers have started to transcend single-molecule applications and to provide a tool for studying in vivo cell-biology questions. Starting out mainly as a tool to sort, manipulate, push, and pull in a qualitative manner, the first reports have appeared in which processes in or around live cells are analyzed using quantitative tweezers measurements, for example for the unraveling of phagocytosis mechanics.3 Such applications of optical tweezers in the field of cell biology have great potential to yield new insights.
With the NanoTracker, JPK’s new experimental life-science platform, compact and off-the-shelf quantitative optical tweezers have become available. The platform lives up to the benchmark standards of single-molecule biophysics. At the same time, it is versatile and user-friendly enough to allow easy operation by researchers unaccustomed to working with lasers and optics.
References
- K. Svoboda, C.F. Schmidt, B.J. Schnapp, and S.M. Block, Nature 365, 721 (1993).
- J.T. Finer, R.M. Simmons, and J.A. Spudich, Nature 368, 113 (1994).
- H. Kress et al., Proc Natl Acad Sci U S A 104(28), 11633 (2007).
Joost van Mameren earned his PhD with a project focused on single-molecule biophysics. He is application scientist at JPK Instruments, Berlin, Germany, www.jpk.com. Contact him at [email protected].