3D OPTICAL IMAGING/SINGLE-MOLECULE LOCALIZATION MICROSCOPY: Extreme precision in 3D: Adaptive optics boosts super-resolution microscopy
ByAudrius Jasaitis, Grégory Clouvel, and Xavier Levecq
Designed to expand the utility of super-resolution optical imaging methods PALM and STORM, a new adaptive optics approach enables the localization of a single emitter in all three dimensions with quasi-isotropic precision of 5–15 nm.
Despite the superb lateral resolution of ~10 nm for photoactivation localization microscopy (PALM) and stochastic optical reconstruction microscopy (STORM), these methods remain two-dimensional.1-3 This is because the point spread function (PSF) is symmetrical along the z-axis and determining whether the point source is located above or below the focus is impossible. Among options for breaking the symmetry of the PSF are inducing astigmatism using a cylindrical lens,4 creating a helical PSF5 and employing the biplane method.6 Using these methods enables achieving z localization precision of approximately 50–100 nm.
To fully benefit from the resolving power of PALM/STORM techniques, it is important to minimize the aberrations that arise in real-life situations. A microscope's components, the refractive index mismatch between the objective and the immersion medium, and the biological sample itself all introduce various types of aberrations. The result is a degradation of the PSF's quality, which leads to a reduction of the number of detected photons and, ultimately, decreased resolution. To address this problem, our research team at Imagine Optic (Orsay, France) has developed a plug-and-play adaptive optics approach. The technique employs a high-quality optical system that includes a wavefront sensor and a deformable mirror. Together, these components measure and correct for aberrations with extreme precision. The result is an increased number of detected photons and an improved lateral localization precision in PALM/STORM methods.
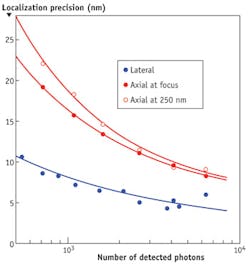
The method can also introduce small amounts of "perfect astigmatism" to provide 3D imaging capability similarly to cylindrical lenses. "Perfect astigmatism" provides a distinct advantage because the deformable mirror introduces astigmatism that is not contaminated by other aberrations, such that have been observed when using cylindrical lenses.7 Its extremely high-quality PSF allows PALM/STORM microscopes to achieve lateral resolutions of 5–8 nm and axial resolutions of 10–16 nm in a typical biological sample and typical conditions with the detection of 4000–1000 photons, respectively (see Fig. 1).
A test of precision
To demonstrate the localization precision of PALM and STORM methods used together with the device, we performed two-color, 3D imaging of a pair of fluorescently labeled centrosomal proteins.
Our PALM/STORM setup was based on a standard epifluorescence microscope (Nikon Ti-E) equipped with an oil-immersion objective (Nikon Apo TIRF 100X, 1.49 NA). Epifluorescence excitation was provided by either a metal-halide lamp or a laser (405 or 561 nm).7 The adaptive optics device, called MicAO 3DSR, was simply inserted into the emission pathway between the microscope and the EMCCD camera (Andor Ixon3 DU897).
For the 3D imaging, we introduced 60 nm root-mean-square (RMS) of perfect astigmatism using the deformable mirror, thereby breaking the symmetry of PSF along the z-axis. As such, it became extended along one direction in x-y plane when the point source was imaged above, and in another direction when below the focal plane (see Fig. 2). This allowed us to determine the location of the fluorophore along the z-axis and construct 3D images. We took a stack of 20,000 images for each color separately. The integration time of each frame was 30 ms and we collected on average 1500 photons/spot. To determine the central position of each spot, we fitted images with two-dimensional Gaussian function using the procedure based on a modified multi target tracking (MTT) algorithm.8
Demonstration outcomes
Michel Bornen's group at the Institute Curie (Paris, France) made the first attempt to do this in two dimensions.9 The challenge of this experiment was to find cells where the centrosome would be properly oriented for the imaging since the angle of the sample with respect to the objective of the microscope was fixed. The authors established a HeLa cell line stably expressing mEos2-Centrin1 to enable the identification of centrioles. They also chose the Cep164 component of distal appendages as a candidate for simultaneous direct STORM (dSTORM) imaging because distal appendages of the mother centrioles are only a few nanometers in width and are challenging to image by immuno-EM. They labeled the HeLa mEos2-centrin1 cell line with an antibody to Cep164, stained it with Alexa 647-conjugated secondary antibody, and performed dual-color 2D PALM/dSTORM imaging.9
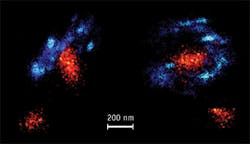
Two different orientations of the centrosome, imaged with two-color 3D PALM/dSTORM using MicAO 3DSR, are shown in Figure 3. In this dataset, the average amount of detected photons from fluorophores was 1500, providing us with 7 nm localization precision in the lateral and 13 nm in axial directions (see Fig. 2). Even at this scale, we still did not see overlapping points between two colors, which further demonstrates the precision of localization in this experiment. A striking observation is that the centrin staining in the mother-centriole appears tilted by about 40 degrees with respect to the distal appendage ring. In addition, in a top view of the mother-centriole (see Fig. 3, right), the centrin concentration seems elongated. This was already observed in similar view in 2D PALM/STORM, but the 3D dataset suggests a more complex spatial distribution of intra-centriolar centrin than expected previously.9 Finally, we observed that the Cep164 ring, which was not complete in this dataset, possibly due to technical reasons, was apparently composed of straight elements with a specific orientation, which could correspond to the distal appendages.
Although much more work is needed with other centrosomal markers, including markers of the centriole wall, our preliminary results demonstrate the power of using PALM/STORM method when it is operated together with MicAO 3DSR.
ACKNOWLEDGEMENT
This work was partially funded by the TRIDIMIC project (Agence nationale de recherche, ANR). We would like to thank James Sillibourne and Michel Bornens (Institute Curie, Paris) for sample preparations. We are grateful to Ignacio Izeddin and Xavier Darzacq (Ecole Normale Supérieure [IBENS], Paris) and Maxime Dahan (Institute Curie, Paris) for their technical support.
REFERENCES
1. E. Betzig et al., Science, 313, 1642–1645 (2006).
2. S. T. Hess, T. P. Girirajan, and M. D. Mason, Biophys. J., 91, 4258–4272 (2006).
3. M. J. Rust, M. Bates, and X. Zhuang, Nat. Meth., 3, 793–795 (2006).
4. B. Huang, J. Wang, M. Bates, and X. Zhuang, Science, 319, 810–813 (2008).
5. S. Quirin, S. R. P. Pavani, and R. Piestun, Proc. Nat. Acad. Sci., 109, 675–679 (2012).
6. M. F. Juette et al., Nat. Meth., 5, 527–529 (2008).
7. I. Izeddin et al., Opt. Exp., 20, 4957–4967 (2012).
8. A. Sergé, N. Bertaux, H. Rigneault, and D. Marguet, Nat. Meth., 5, 687–694 (2008).
9. J. E. Sillibourne et al., Cytoskeleton, 68, 619–627 (2011).
AUDRIUS JASAITIS, PhD, is microscopy business development manager, GRÉGORY CLOUVEL is R&D engineer in adaptive optics for microscopy, and XAVIER LEVECQ, PhD, is VP and chief research officer with Imagine Optic (Orsay, France), www.imagine-optic.com. Contact Dr. Jasaitis at [email protected].