OPTOACOUSTICS/DEEP TISSUE IMAGING: Photoacoustic tomography is ready to revolutionize
Lihong V. Wang and Song Hu
EDITOR'S NOTE: This article is adapted and updated from a review paper—L. V. Wang and S. Hu, Science, 335, 1458–1462 (2012)—with permission from AAAS.
Optical imaging is powerful in that it allows distinguishing of biological structures based on chemical composition. Purely optical methods—which form images based on the passage of light through tissue—fall into two categories, both of which involve significant restrictions: Ballistic (minimally scattered) optical microscopy provides fine resolution, but is severely limited in terms of depth (up to only ~1 mm, as defined by the optical diffusion limit).1,2 Diffuse (multiscattered) optical tomography can probe centimeters into tissue, but offers spatial resolution of only about one-third of the imaging depth.3
Fortunately, photons in tissue can be converted into ultrasonic waves, which scatter much less4 (about three orders of magnitude less than optical scattering per unit path length) and thus enable tissue imaging at greater depths. This is the basis of photoacoustic tomography (PAT), which enables imaging of living biological structures in high resolution on multiple scales using the same contrast (e.g., anatomical, functional, metabolic, molecular, and genetic contrasts of vasculature, and hemodynamics, oxygen metabolism, biomarkers, and gene expression). In fact, PAT's scalability provides an unprecedented opportunity to seamlessly follow a complex biological system from micro to macro (or vice versa)—which promises to help explain and even predict biological phenomena at multiple scales.
The photoacoustic effect is enabled when heat, generated by light-absorbing molecules, launches ultrasonic waves by boosting pressure—which detectors can read to create images. PAT allows scaling of spatial resolution according to desired imaging depth, while maintaining a high depth-to-resolution ratio: As a rule of thumb, the achievable spatial resolution is approximately 1/200 of imaging depth, up to 8.4 cm.5 Moreover, PAT provides inherently background-free detection because photoacoustic amplitude is proportional to optical absorption (nonabsorbing components present no background). And by exciting different molecules at different optical wavelengths, PAT reveals rich optical contrasts according to chemical composition.
PAT adds to the capabilities of other modalities: It images optical absorption with 100% sensitivity,6 two orders of magnitude greater than do confocal microscopy and optical coherence tomography (OCT).7 Unlike fluorescence imaging, PAT ensures no leakage of excitation photons into detectors and, unlike OCT and ultrasonography, it is speckle-free.8 While all molecules are optically absorbing at some wavelengths and can potentially be imaged by PAT, far fewer molecules are fluorescent. Finally, conventional ultrasound imaging measures only mechanical contrasts, while PAT measures optical and thermoelastic contrasts.
PAT has been developed rapidly in the past decade, with applications explored in vascular biology,9,10 oncology,11,12 neurology,13-15 ophthalmology,16,17 dermatology,18,19 gastroenterology,20,21 and cardiology.22,23 The technique uses laser energy to trigger thermal and acoustic impulse responses. A nanosecond-pulsed beam is typically used, although intensity-modulated light sources may also be used. Currently, PAT has three major implementations: focused-scanning photoacoustic microscopy (PAM), photoacoustic computed tomography (PACT), and photoacoustic endoscopy (PAE). Whereas PAM and PAE usually aim to image millimeters deep at micrometer-scale resolution, PACT can be implemented for both microscopic and macroscopic imaging.
Photoacoustic microscopy (PAM)
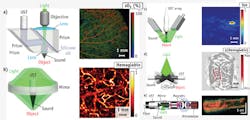
In PAM, both optical excitation and ultrasonic detection are focused, and the dual foci are usually configured confocally to maximize sensitivity. Each laser pulse produces a one-dimensional (1D), depth-resolved image without mechanical scanning, and 2D transverse scanning generates a 3D image. Axial resolution is determined by the acoustic time of flight, whereas lateral resolution is determined by the overlap of the dual foci. Quantitatively, the axial and lateral resolutions are defined as the corresponding full-widths at half-maximum (FWHM) of the system response to a point target. Depending on whether the optical or ultrasonic focus is finer, PAM is further classified into optical-resolution (OR-PAM)25,26 and acoustic-resolution (AR-PAM) varieties.27
OR-PAM provides lateral resolution at the subcellular or cellular scale ranging from a few hundred nanometers to a few micrometers (see Fig. 1a). If such resolution were to be achieved acoustically, the center frequency of the acoustic signal would have to be at least 300 MHz. At such a high frequency, ultrasonic waves sustain severe propagation loss and can penetrate only a few hundred micrometers in tissue. Fortunately, optical focusing can readily confine the photoacoustic excitation for high lateral resolution while maintaining substantial imaging depth; in addition, acoustic focusing can improve detection sensitivity. This system enables in-vivo, label-free functional imaging of hemoglobin oxygen saturation (sO2) in vessels down to single capillaries. However, imaging depth is limited by optical diffusion to 1.2 mm in-vivo.25
In OR-PAM, the laser beam is focused by a microscope objective to a diffraction-limited spot for excitation in the tissue. An optical-acoustic beam combiner, consisting of two prisms sandwiching a thin layer of silicone oil, is positioned beneath the objective to align the optical excitation and acoustic detection coaxially and confocally. The matched optical refractive indices—but mismatched acoustic impedances—between the prism glass and silicone oil provide optical transmission and acoustic reflection. The optical aberration created by the optical transmission through the beam combiner is offset by a correction lens attached to the top surface of the right-angle prism. To focus acoustic detection, a concave acoustic lens is ground into the bottom of the rhomboid prism. An unfocused ultrasonic transducer with a broad bandwidth matching that of the received acoustic waves is attached to the top of the rhomboid prism. Although ideal for light transmission, the solid-liquid interface adversely transforms 85% of the incident acoustic energy from longitudinal waves to shear waves. Because shear waves are not detected with high sensitivity, the rhomboid prism is used to regain the longitudinal wave at the second inclined surface.
At depths beyond the optical diffusion limit and up to a few millimeters, AR-PAM achieves high resolution by taking advantage of relatively low acoustic scattering (see Fig. 1b). Despite diffuse optical excitation, lateral resolution of tens of micrometers is achieved by diffraction-limited acoustic detection. In AR-PAM, optical excitation is implemented through dark-field illumination for two critical reasons: First, donut-shaped illumination eliminates otherwise dominant interference signals from the tissue surface, and second, the donut hole is ideal for positioning the ultrasonic transducer coaxially and confocally, with respect to the optical excitation. The system provides 45 μm lateral resolution in-vivo with a 3 mm imaging depth. Anatomical images of the human cutaneous microvasculature in both the superficial epidermis and deep dermis have been acquired by detecting hemoglobin.19 However, further advancement of the imaging depth to centimeters for macroscopic imaging requires the use of more energetic lasers at low pulse repetition rates—which means that transverse scanning is too slow for many clinical applications.
Photoacoustic computed tomography (PACT)
To accelerate data acquisition, state-of-the-art ultrasonic array detectors have been used for PACT. The entire region of interest (ROI) is excited by an expanded optical beam, and photoacoustic waves are simultaneously detected by an ultrasonic array. Then, an inverse algorithm—essentially a method for sophisticated triangulation of photoacoustic sources from the time-resolved acoustic signals—is used to reconstruct a high-resolution image.28-31 As most ultrasonic arrays are 1D, the 2D resolutions in the imaging plane are derived from reconstruction, whereas the orthogonal resolution comes from cylindrical acoustic focusing. The imaging plane can be further translated along the orthogonal dimension for 3D imaging. According to the anatomy of the organ of interest, the ultrasonic array may be configured linearly32 or circularly.33-35
In linear-array PACT, a multimode optical fiber bundle is bifurcated to flank the handheld ultrasonic array for dark-field optical illumination (see Fig. 1c). A single laser pulse—with a safe exposure to the tissue (≤20 mJ/cm2 in the visible spectral range)—yields a 2D image. A clinical ultrasound imaging system has been adapted for concurrent imaging with PACT. This system, with an axial resolution of 400 μm and a lateral resolution of ~1 mm,36 has been used for noninvasive in-vivo functional imaging of methylene blue-labeled sentinel lymph nodes in small animals, and more recently in human breast cancer patients.37
Circular-array PACT is designed to accommodate roundish objects, such as the brain, a peripheral joint, and even the whole body of a small animal (see Fig. 1d). The ROI is encircled by the array to detect photoacoustic waves propagating along all in-plane directions; unlike the partial-view detection (i.e., the angle subtended by the ultrasound detectors with respect to the object is less than 360°) in linear-array PACT, full-view detection provides high-quality images without missing boundaries.38 The principle of circular-array PACT was originally demonstrated by circularly scanning a single-element ultrasonic transducer in the first functional PAT system, which imaged the cerebral vascular response to one-sided whisker stimulation in an adult rat through intact scalp and skull with an in-plane resolution of ~200 μm.15
Photoacoustic endoscopy (PAE)
PAE has been intensively investigated in recent years20-23, 39 as a means of imaging internal organs such as the esophagus and colon. In a representative PAE design, light from a high-repetition-rate laser is delivered by a multimode optical fiber placed in the central hole of a ring transducer (see Fig. 1e).20 An optically and acoustically reflective mirror, driven by a micromotor through coupled magnets, rotates both the optical illumination and the acoustic detection for circumferential cross-sectional scanning. Further, a linear motor pulls back the entire probe for volumetric imaging. In contrast to conventional optical endoscopy, which has an imaging depth within the optical diffusion limit, PAE has shown a 7 mm imaging depth.21
Multiscale
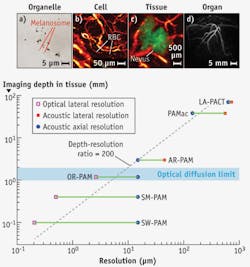
The elegant marriage between light and sound endows PAT with the unique capability of scaling its spatial resolution and imaging depth across both optical and ultrasonic dimensions. The lateral resolution of OR-PAM is given by RL,OR = 0.51 λ/NA, where λ denotes the optical wavelength and NA is the numerical aperture of the microscope objective. Varying the NA can scale the lateral resolution from subwavelength to a few wavelengths, with the imaging depth varied accordingly. With NA = 1.23 and λ = 532 nm, a 220 nm lateral resolution has been achieved with an imaging depth of 100 μm, enabling in-vivo subcellular imaging of individual melanosomes (see Fig. 2a).40 Halving the NA to 0.63 quadruples the imaging depth while the lateral resolution is still maintained at 500 nm.41 Reducing the NA to 0.1 further triples the imaging depth to the optical diffusion limit and relaxes the lateral resolution to 2.6 μm, enabling in-vivo label-free functional imaging of individual red blood cells flowing in capillaries (see Fig. 2b).25 As in conventional optical microscopes, it is possible to combine multiple optical objectives of different NAs in a single OR-PAM system, which would allow convenient adjustment of the magnification. Further, OR-PAM and AR-PAM systems can be integrated to extend the range of scalability of a single device.
The lateral resolution of AR-PAM or partial-view PACT is given by RL,AR = 0.71 vs/(NA f0), where vs is the speed of sound, NA is the acoustic numerical aperture, and f0 is the photoacoustic center frequency. The center frequency f0 is determined by the laser pulse width, the targeted tissue depth, and the ultrasonic transducer's frequency response. With f0 of 50 MHz and NA of 0.44, AR-PAM has achieved a lateral resolution of 45 μm and imaging depth to 3 mm.27 Such a system is adequate to see through human skin lesions in-vivo, as required for accurate diagnosis and staging (see Fig. 2c).19 Reducing the center frequency to 5 MHz extends the imaging depth to 4 cm and relaxes the lateral resolution to 560 μm.42 Because the resolution is now within the resolving power of human eyes, such an instrument is called a photoacoustic macroscope (PAMac). A PACT system based on a clinical linear ultrasound array operating with a frequency band of 4–8 MHz has extended the imaging depth to 8.4 cm,5 with a submillimeter lateral resolution (720 μm).37 PACT can image breast vasculature in-vivo (see Fig. 2d),34 and can also perform microscopic imaging when operating at high ultrasonic frequencies.43The axial resolution of PAM or partial-view PACT always originates from the time of arrival of the acoustic signal. It can be estimated as RA = 0.88 vs/Δf, where Δf is the photoacoustic bandwidth (approximately proportional to f0). So far, axial resolutions ranging from 15 to 640 μm have been achieved in PAT systems of various targeted imaging depths.26,37 The 2D in-plane resolutions of full-view PACT can be similarly estimated with Δf.
The optimal tradeoff between spatial resolution and imaging depth in PAT depends on the application (see Fig. 2e). Within the optical diffusion limit, the penetration of OR-PAM is approximately proportional to the chosen lateral resolution. Beyond the limit, the imaging depth is primarily determined by the frequency-dependent acoustic attenuation. As both f0 and Δf are inversely proportional to the desired imaging depth, the lateral and axial resolutions are proportional to the imaging depth. For both regimes, the ratio of the imaging depth to the best spatial resolution is roughly a constant of 200, making PAT a high-resolution modality across all four length scales.
Multicontrast
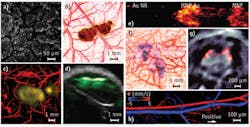
With selected optical wavelengths, PAT can probe a wide variety of endogenous or exogenous absorbers to reveal the anatomy, function, metabolism, and molecular/genetic processes in biological systems in-vivo. Endogenously, DNA/RNA, hemoglobin, melanin, water, and lipid are important anatomical and functional contrast agents. Using the strong ultraviolet absorption of DNA and RNA, OR-PAM recently achieved noninvasive imaging of individual cell nuclei, which can provide an in-vivo label-free substitute for ex-vivo hematoxylin and eosin staining histology (see Fig. 3a).44 Because malfunction of DNA replication induces abnormal nuclear morphology in cancer, this technology can potentially provide early detection and intraoperative demarcation of cancer.Hemoglobin, as a primary oxygen carrier, is essential to tissue metabolism. Using hemoglobin's predominant optical absorption in the visible range over other absorbers, PAT provides comprehensive anatomical and functional imaging of the blood circulation system.9,45 Abnormal concentrations of water and lipid can be important disease indicators. Their relatively strong optical absorption in the near-infrared (NIR) range allows PAT to map their distributions at substantial depths in-vivo.46,47 Melanin, a major pigment in the skin and most melanomas, has broadband optical absorption from the ultraviolet to the NIR range, which PAT can spectroscopically distinguish from hemoglobin absorption. Simultaneous imaging of the melanoma anatomy and the surrounding vascular function provides unprecedented opportunity for understanding the interactions between a tumor and its microenvironment and for noninvasively detecting and staging melanoma (see Fig. 3b).27
Exogenous contrast agents further extend PAT to molecular and genetic imaging—nanoparticles, organic dyes, and reporter gene products can be excellent photoacoustic contrast agents. The primary advantage of gold nanoparticles lies in their large absorption cross-section tuned to the optical window (~730 nm), minimizing endogenous absorption and maximizing imaging depth. Moreover, the bioconjugation capability of nanoparticles enables effective biomarker targeting for both molecular imaging12,48 and drug delivery (see Figs. 3c and 3d). Recently, the use of iron oxide and gold-coupled core-shell nanoparticles as a photoacoustic contrast agent has led to the development of magnetomotive PAT (see Fig. 3e),49 which markedly improves the contrast and specificity of PAT by suppressing the non-magnetomotive background. Depending on the application, the relatively slow tissue clearance of nanoparticles can be either an advantage or a disadvantage.
Although the clinical translation of most nanoparticles is still awaiting FDA approval, many organic dyes have been approved for human use. Organic dyes clear rapidly from the body because of their small molecular size (typically ~1 nm), and some can penetrate the blood-brain barrier. Reporter gene products can be detected for PAT of biological processes at the genetic level, as was demonstrated using the LacZ gene—a common reporter encoding the protein β-galactosidase.50 Gliosarcoma cells transfected with LacZ genes were inoculated into a Sprague-Dawley rat. As the tumor grew, LacZ genes were expressed to β-galactosidase, which metabolized the locally injected lactose-like substrate into highly absorbing blue products, thereby providing contrast for genetic PAT in-vivo (see Fig. 3f).50 PAT has imaged even fluorescent proteins from reporter genes in-vivo (see Fig. 3g).51
PAT can potentially image all molecules at appropriate wavelengths, whereas only a small subset of molecules is fluorescent. Even fluorophores can serve as absorbing contrast agents for PAT.14,51,52 Optical excitation of fluorophores, in the absence of photochemical relaxation, relaxes via either fluorescence or thermal emission. Because most fluorophores have imperfect fluorescence quantum yields, PAT can rely on the thermal relaxation for high-resolution deep imaging of fluorophores.
Besides such static contrasts, PAT can also image two important dynamic contrasts: blood flow (hemodynamic) and temperature variation (thermodynamic). The recently discovered photoacoustic Doppler effect laid the foundation for PAT of flow (see Fig. 3h).53,54 With excellent scalability, Doppler PAT bridges the spatial gap between scattering-based optical and ultrasonic technologies. More important, the high optical absorption contrast between the intravascular blood and extravascular background greatly increases detection sensitivity.
Tissue temperature monitoring is essential for thermal therapy. Because the initial photoacoustic pressure depends on the equilibrium temperature, PAT provides a potential means for high-resolution temperature imaging deep in tissue.55,56 Recent tissue phantom experiments showed that the initial photoacoustic pressure increases with the equilibrium temperature by ~5% per degree, which enables a sensitivity of about 0.1°C.55
Combining both static and dynamic contrasts from PAT enables metabolic imaging. Indeed, PAT is the only modality that uses endogenous contrasts to measure all the parameters—including vessel diameter, total hemoglobin concentration, sO2, tissue volume of interest, and blood flow velocity—required to compute the metabolic rate of oxygen (MRO2). Recent work demonstrated label-free absolute quantification of the MRO2 in a living mouse.45
The future of PAT
PAT is expected to find broad applications in biology and medicine. Major preclinical applications include imaging of angiogenesis, microcirculation, tumor microenvironments, drug response, brain functions, biomarkers, and gene activities. Initial clinical applications include melanoma cancer imaging, gastrointestinal tract endoscopy, intravascular catheter imaging, neonatal brain imaging, breast cancer detection, prostate cancer detection, guided sentinel lymph node needle biopsy for cancer staging, early chemotherapeutic response imaging, dosimetry in thermal therapy, in-vivo label-free histology, blood perfusion imaging, blood oxygenation imaging, and tissue metabolism imaging. Although preclinical PAT systems have been commercialized, clinical systems need to pass rigorous validation and arduous regulatory approval.
PAT is capable of in-vivo metabolic imaging based only on endogenous contrast. Upscaling metabolic PAT from small animals45 to humans is expected to revolutionize the screening, diagnosis, and treatment of metabolic diseases, particularly cancers and cerebral disorders. Downscaling to cells provides a tool for understanding metabolic pathways. Because hypermetabolism is a quintessential hallmark of cancer, metabolic PAT may enable in-vivo screening at the earliest stage without contrast agents.
The scalability of PAT provides an unprecedented opportunity to link a complex biological system at multiple length scales through consistent optical absorption contrasts. Currently, correlation of microscopic and macroscopic images can be challenging because of their vastly different contrast mechanisms. Experimental observations from multiscale PAT are expected to facilitate the development of theoretical models for systems biology that explain and even predict biological phenomena at multiple scales. Moreover, PAT will likely accelerate the translation of microscopic laboratory discoveries to macroscopic clinical practice.
Help wanted
To maximize its impact in biomedicine, however, PAT must overcome multiple technical challenges. For instance, high-speed multicontrast PAM or PAE based on spectroscopy will require the development of high-repetition lasers with fast wavelength-tuning at each scan position. Also, PAE probes must be further miniaturized to fit into existing endoscopes and even intravascular catheters.
For deep-penetrating PACT, high-energy lasers with video-rate pulse repetition are needed. The required laser energy, however, can potentially be lowered by using time-reversed ultrasonically encoded (TRUE) optical focusing to improve light penetration.57 Also needed are sophisticated algorithms to perfect molecular quantification and to suppress skull-induced artifacts.
Such developments will enable PAT to revolutionize both fundamental life sciences and clinical patient care.
REFERENCES
For the list of references, please visit https://www.laserfocusworld.com/14214607.
Lihong V. Wang holds the Gene K. Beare Distinguished Professorship of Biomedical Engineering, and Song Hu is a postdoctoral scholar at the Optical Imaging Laboratory at Washington University in St. Louis, http://oilab.seas.wustl.edu. Contact Dr. Wang at [email protected].