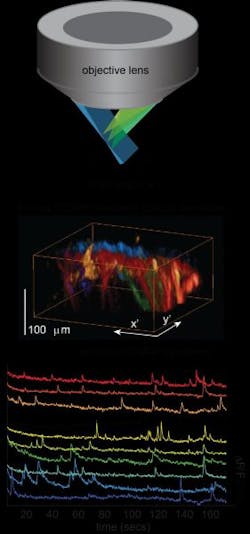
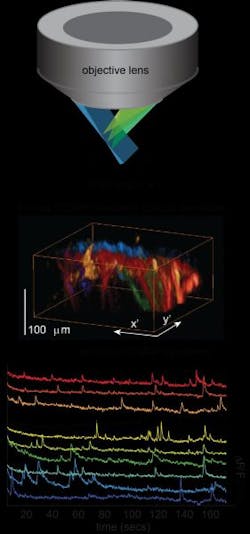
High-speed 3D microscopy approach images live, freely moving samples
Elizabeth Hillman, associate professor of biomedical engineering at Columbia Engineering and of radiology at Columbia University Medical Center (CUMC; both in New York, NY), has developed a new microscope that can image living things in 3D at very high speeds. In doing so, she has overcome some of the major hurdles faced by existing technologies, delivering 10 to 100 times faster 3D imaging speeds than laser scanning confocal, two-photon, and light-sheet microscopy. Hillman's new approach, dubbed swept confocally aligned planar excitation microscopy (SCAPE), uses a simple, single-objective imaging geometry that requires no sample mounting or translation, making it possible to image freely moving, living samples.
Related: Multispectral system enables brain development discovery
"The ability to perform real-time 3D imaging at cellular resolution in behaving organisms is a new frontier for biomedical and neuroscience research," says Hillman, who is also a member of Columbia's Mortimer B. Zuckerman Mind Brain Behavior Institute. "With SCAPE, we can now image complex, living things, such as neurons firing in the rodent brain, crawling fruit fly larvae, and single cells in the zebrafish heart while the heart is actually beating spontaneously—this has not been possible until now."
Highly aligned with the goals of President Obama's BRAIN Initiative, SCAPE is a variation on light-sheet imaging. While conventional light-sheet microscopes use two awkwardly positioned objective lenses, Hillman realized that she could use a single-objective lens, and then that she could sweep the light sheet to generate 3D images without moving the objective or the sample. "This combination makes SCAPE both fast and very simple to use, as well as surprisingly inexpensive," she explains. "We think it will be transformative in bringing the ability to capture high-speed 3D cellular activity to a wide range of living samples."
While SCAPE cannot yet compete with the penetration depth of conventional two-photon microscopy, Hillman and her collaborators have already used the system to observe firing in 3D neuronal dendritic trees in superficial layers of the mouse brain. In small organisms, including zebrafish larvae, SCAPE can see through the entire organism. By tracking these tiny, unrestrained creatures in 3D at high speeds, SCAPE can capture both cellular structure and function and behavior. SCAPE can also be combined with optogenetics and other tissue manipulations during imaging because, unlike other systems, it does not require any movement of the imaging objective lens or the sample to create a 3D image.
Beyond neuroscience, Hillman sees many future applications of SCAPE, including imaging cellular replication, function, and motion in intact tissues, 3D cell cultures, and engineered tissue constructs, as well as imaging 3D dynamics in microfluidics and flow cytometry systems. She also plans to explore clinical applications of SCAPE such as video-rate 3D microendoscopy and intrasurgical imaging. Next-generation versions of SCAPE are in development that will deliver even better speed, resolution, sensitivity, and penetration depth.
Hillman's technology is available for licensing from Columbia Technology Ventures and has already attracted interest from multiple companies.
Full details of the work appear in the journal Nature Photonics; for more information, please visit http://dx.doi.org/10.1038/nphoton.2014.323.
-----
Follow us on Twitter, 'like' us on Facebook, connect with us on Google+, and join our group on LinkedIn
Subscribe now to BioOptics World magazine; it's free!