Novel OCT method shows differences underlying the airway responses of asthma patients
Seeking to understand why some individuals allergic to airborne allergens develop asthma and others do not, researchers at Massachusetts General Hospital (MGH; Boston, MA) used optical coherence tomography (OCT) imaging and other novel approaches to identify some key differences in the immune response and the sensitivity of airway cells to inflammation between allergic individuals with and without asthma.
Related: NIH awards $1.4M grant to Mass General's Center for Biomedical OCT Research and Translation
"Our study found that, despite similar levels of systemic allergy response, allergen-specific CD4 helper T cells in individuals with asthma had a more potent response to airway allergens than did allergic individuals without asthma," says Andrew Luster, MD, Ph.D., of MGH's Center for Immunology and Inflammatory Diseases (CIID) and chief of the Division of Rheumatology, Allergy and Immunology, who led one research team along with Benjamin Medoff, MD, chief of the MGH Division of Pulmonary and Critical Care Medicine. "Second, the airway cells of asthmatic individuals were both structurally different and have a greater response to allergens and to allergy-associated inflammation."
Several of the findings of Luster's team depended on the ability to analyze, in living patients, the structure and function of the airway smooth muscle cells that contract and obstruct the airway during an asthma attack. This was made possible by an advanced version of OCT developed by the other team, led by Melissa Suter, Ph.D., of the MGH Division of Pulmonary and Critical Care Medicine.
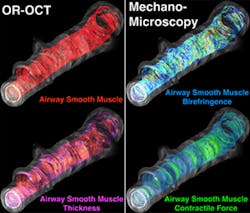
"Although in theory OCT has the resolution necessary to see airway smooth muscle, it doesn't provide sufficient contrast against the surrounding airway tissues to do so," Suter says. "Standard OCT generates images by measuring the amount of light that is reflected back from tissue, but there is more information in the returning light than just how much is reflected back. When light travels through organized tissue, which smooth muscle is, different aspects of the light will travel at different speeds depending on the orientation of the tissues. The ability to measure orientation-dependent properties, like light polarization, lets us know how airway smooth muscle is organized."
Suter's team developed a microscopic imaging platform that provides both orientation-resolved OCT (OR-OCT) images, which reveal the amount of airway smooth muscle, and mechano-microscopy, which measures the force with which muscle tissue contracts. After validating the ability of their equipment to do so in segments of animal airway, they collaborated with Luster's group to assess the structure of airway smooth muscle in six volunteer study participants—three with mild allergic asthma and three with no allergy—and found that airway smooth muscle was two times thicker in participants with asthma.
Luster's study used Suter's technology and several other approaches to investigate differences in the airway response to allergens among 36 participants with mild allergic asthma; 48 with allergies, but no history of asthma; and five healthy control participants. After initial measurements of participants' airway smooth muscle using OR-OCT, researchers introduced diluted amounts of the appropriate allergen—either cat dander or dust mites—into a small section of participants' lungs. They then measured numerous aspects of the immune response within the lungs, with particular attention to inflammation and to characteristics of the CD4 T cells that specifically respond to the allergen in question.
Both allergic patients with and those without asthma responded to the allergen challenge with type 2 inflammation, the type that is characteristic of allergy, and both groups were found to have similar levels of several types of T cells. But use of a novel tool developed in the laboratory of James Moon, Ph.D., in the MGH CIID—an immunologic agent that recognizes allergen-specific T cell receptors—allowed the team to identify and analyze allergen-specific CD4 T cells in participants' airways. While both participants with and those without asthma had increases in these allergen-specific CD4 T cells after the allergen challenge, cells in participants with asthma showed markedly higher expression of two receptors for type 2 innate immune signals.
A collaborator with Luster's team, Mehmet Kesimer, PhD, from the University of North Carolina at Chapel Hill, analyzed both the amount and the consistency of mucus secreted in participants’ airways in response to the allergen challenge, finding in asthmatic participants both larger amounts of mucus and increased levels of a protein that makes mucus more gelatinous and may increase airway hyper-reactivity. Use of OR-OCT to measure both the thickness of airway smooth muscle and the width of the bands of muscle that wrap around the airway showed significantly greater smooth muscle mass in participants with asthma than in either allergic participants without asthma or in healthy controls.
Luster, who is the Harrison Professor of Medicine at Harvard Medical School (HMS), explains that the ability to understand the mechanisms that determine the sensitivity of the epithelial and smooth muscle cells of the airway to inflammation in asthma could someday help to identify novel targets to treat or reverse asthma, or even to prevent its development in allergic individuals.
Full details of the work appear in the journal Science Translational Medicine; for more information, please visit http://dx.doi.org/10.1126/scitranslmed.aag1424.