CELL BIOLOGY : Light-sheet microscopes reveal cells in action
Early in the 20th century, the University of Heidelberg established itself as a pioneering center for interdisciplinary studies. A century later, the medieval German town hosts a new center for cross-disciplinary research. The main unit of the European Molecular Biology Laboratory (EMBL) based there undertakes research in molecular biology that involves tools and technologies from physics, chemistry, and other related fields. Typical of that interdisciplinary approach is the work of Ernst Stelzer’s light microscopy group. The team applies optical technologies to the visualization and manipulation of living cells and embryos in two, three, and even four dimensions. “What motivates the group is working with things that are physiologically relevant,” Stelzer explains. “Essentially, we think about the problems first, and then think about what kind of techniques will help us to solve them.”
null
Cell biologists face a particular problem when they try to view single cells in live tissues. Conventional optical microscopy yields only two-dimensional images that lose much of the context in which living cells operate. Two factors compromise efforts to image an extra dimension: specimens both scatter and absorb light, and phototoxicity often kills cells under observation. To address those problems, the Stelzer team has developed fluorescence microscopes based on light sheets. The light sheets are fed into specimens from the side and observed at right angles to the illumination optical axis. “The idea of uncoupled detection at a 90° angle is most fundamental,” explains team member Philipp Keller. “You arrive at an optical sectioning technique by focusing on a single plane. So you don’t create anything out of focus and you don’t waste any photons.”
The team has created two varieties of its microscope. The single-plane illumination microscope (SPIM) images cells in three dimensions, while the digital scanned light sheet fluorescence microscope (DSLM) permits researchers to record and track cellular images over periods of time. “These novel microscopes take advantage of modern camera technologies and can be combined with essentially every contrast and specimen manipulation tool,” Stelzer explains. “However, they operate in a truly three-dimensional fashion.”
The group started to develop its microscopes in the mid 1990s. “We looked at highly scattering objects,” Stelzer recalls. “We realized that we had the software to reconstruct them, but when the objects were optically dense it didn’t work.” Having first worked on a confocal microscope, the group started to develop a SPIM system in 2002. “This is the technique for looking in three dimensions,” Stelzer says. “We have a series of papers on microtubule dynamics. The results are really interesting; they could change the field.”
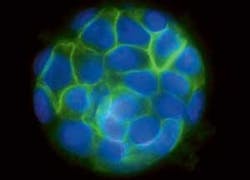
In the most recent example of the value of SPIM microscopy, group member Emmanuel Reynaud took images of cysts consisting of human kidney cells (see Fig. 1). The team grows the cysts, each of which consists of about 50 cells, in three dimensions. That permits the cells to communicate with their neighbors just as they do in real life. So the resulting images are notably more representative of real-life cellular behavior, shapes, and arrangements than is the case for samples imaged with traditional two-dimensional microscopes.
What comes after three-dimensional images? Obviously a fourth dimension, in the form of moving pictures. “When we wrote the patents for the SPIM, I immediately thought of using scanners to generate light sheets,” Stelzer says. The thought led to the DSLM system. “We create the light sheet [using an argon-krypton gas laser] in a different way,” Keller explains. “We rely on higher-quality optics and move the single line using a laser scanner. During that time the camera detects the fluorescence emitted along each line.” The methodology has an added advantage beyond enabling researchers to capture images over time. ”It is especially suitable for large specimens,” Keller says. “You can do quantitative imaging of those specimens in their entirety.”
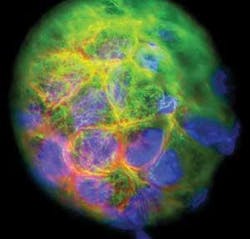
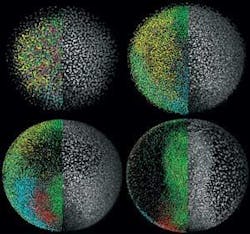
The technology has proved its success in a remarkable set of movies that track the development of a zebrafish embryo over the course of 24 hours (see Fig. 2). During that time, the embryo develops a recognizable backbone and heart and shows signs of creating other major organs. According to Science, which published the group’s report of the advance online, the technique promises to open new windows into the development of embryos and tissues in a wide assortment of species. “We have applied the technique to many other species, including mouse and chicken,” Keller says. “We are talking about very small structures; the same microscope is suitable for all types of structures.”
The group doesn’t plan to stop at movie-making. “The technology has huge potential,” Stelzer points out. “We are continuing to build other variations. And we want to integrate techniques from conventional microscopy such as fluorescence lifetime imaging microscopy into a light sheet-based microscope. The big advantage is that you can look at millions of pixels in parallel, avoiding the bad signal-to-noise ratio of conventional techniques.” – PG