OPTICAL COHERENCE TOMOGRAPHY/BIOIMAGING: One decade and $500M: The impact of federal funding on OCT—Part 1
ERIC A. SWANSON
Since the first work on optical coherence tomography (OCT) in the late 1980s and early 1990s, dozens of companies and hundreds of research groups have been formed, thousands of research articles have been published, and approximately 100 million patients have been scanned with OCT. Meanwhile, hundreds of millions of venture capital and corporate R&D dollars have been invested, hundreds of millions more have gone into company acquisitions, and billions have been generated in revenue. In addition, OCT has created many thousands of man-years of direct jobs (at system and subsystem companies) and probably 10 times that number in indirect jobs.
Governments around the world have supplied critical R&D funding in both basic science and technology and applications, including clinical trials. Analysis of funding data from the past decade highlights some interesting trends—although collecting and compiling it is not easy. Of the hundreds (or perhaps thousands) of government OCT funders that exist (including various countries such as Japan, Australia, China, and Russia; and individual U.S. states, European countries, and Canadian provinces), this report pulls data from 10 sources in the U.S., United Kingdom (UK), Canada, and European Union (EU). Thus, the field of OCT has received more funding than I’ve been able to include in this report.
Defining what constitutes OCT funding is also a challenge. Funding reported in this article represents grants whose title, abstract, or summary mentions “optical coherence tomography.” Thus, this analysis includes grants for developing OCT technology, and those in which OCT serves as a tool for basic science and clinical investigations. As such, it probably represents an expansive view because in several cases OCT is mentioned in abstracts or summaries of clinical trials—particularly in ophthalmic work—but is not the central focus.
A couple of caveats are worth mentioning: Criteria that often accompany funding from government agencies include matching fund requirements, overhead charges (which can vary from 0 to nearly 50%), and allocation of principal investigators’ (PIs’) salaries. Thus, dollars cannot necessarily be equated between agencies. This article does not delve into these issues. Also, data from government sources does not always agree with data provided by PIs; in some cases, PIs have received substantially more than what is listed in this article.
Investments, internationally
Funding—for both academic and for-profit (e.g. SBIR) entities—has grown dramatically overall since the year 2000 (see Fig. 1). National Institutes of Health (NIH) investment has grown impressively from about $5M in 2000 to $65M in 2010, while funding from the National Science Foundation (NSF), the UK, and Canada has fluctuated from year to year—although UK and Canadian data show more of an uptrend compared with that of the NSF, which indicates a downward trend. Total NIH funding in 2010 was up 25% over 2009. As of March 2011, NIH’s OCT-related funding was $336M, whereas NSF’s OCT funding was about $15M and OCT funding from the UK’s Engineering and Physical Sciences Research Council (EPSRC), Biotechnology and Biological Science Research Council (BBSRC), and Medical Research Council (MRC) totaled a similar figure at $19M (though MRC data goes back only to 2005). Canada’s total—about $7M—includes totals from Natural Sciences and Engineering Research Council (NSERC), Canadian Institutes of Health Research (CIHR), and National Cancer Institute of Canada (NCIC), but not from the National Research Council of Canada (NRCC, a major contributor to OCT research) and various federal and provincial sources. While the data sources distributed funds over the duration of the program for some of the larger grants, for others all funding was allocated in a single year—a fact that accounts for some of the odd profiles in the yearly funding plots.
It is important to note that the NIH and other funding figures do not include all the intramural research on OCT-related topics—which for the NIH alone could easily exceed 10% ($34M) over this period. In addition, many other U.S. government agencies fund internal or external OCT research: Such agencies include the Departments of Defense (DOD) and Energy (DOE) labs, Defense Advanced Research Projects Agency (DARPA), Air Force Office of Scientific Research (AFOSR), National Institute of Standards and Technology (NIST), National Aeronautics and Space Association (NASA), Federally Funded Research and Development Centers (FFRDC), among others. For example, the Medical Free Electron Laser Program had requirements that gave substantial support to various university biophotonics research efforts, and a program run out of AFOSR provided more than $40M for biomedical optics during the past decade, much of it related to OCT (and a very large fraction supporting novel work at Massachusetts General Hospital). On the basis of known OCT-related projects, I estimate that the total of external and internal U.S. OCT-related funding from these sources over the past decade may exceed $100M. Thus, total U.S. government funding could easily have surpassed $500M during the past decade, and the worldwide total would be substantially greater.
Assuming much of the NIH’s OCT funding targets large, expensive multi-institution clinical trials, it seems clear that the U.S. may lag in funding for basic OCT science and technology. A look at the NIH sub-agency breakdown reveals that The National Eye Institute (NEI), National Cancer Institute (NCI), and National Institute of Biomedical Imaging and Bioengineering (NIBIB) have been the top three funders, with $131M, $52M, and $42M in grants, respectively (see Fig. 2). Not surprisingly, the NEI leads the pack (representing almost 40% of funding): Ophthalmology is the most mature OCT application, and numerous clinical trials are directly or indirectly related to its use (the fact that some NEI grants are not focused on OCT, but only use or cite OCT does skew the data). What may be surprising is that while cardiology and cancer represent large fractions of the NIH budget, they represent a small fraction of NIH OCT spending. The reason is that commercial cardiovascular and oncology products have only recently received FDA clearance, and the application of OCT in these fields is still new. However, the percentage of NIH expenditure may increasingly favor these larger clinical areas.The recipients of funding
A basic search of government databases produces a list of researchers who have received funding from NIH, NSF, EPSRC/BBRSC/MRC, and NSERC/CIHR/NCIC. Keep in mind that only the point-of-contact organization and PI is listed, though often these funds go to multiple investigators and institutions—so the figures indicated do not necessarily represent that received by the institution or investigator noted. This is especially true for the large NSF grants, and NIH grants that sponsored ophthalmic clinical trial oriented studies—which can involve half a dozen or more institutions collecting data over an extended period in order to perform clinical studies. NSF biomedical engineering programs typically provide smaller amounts of support, except in cases where multiple investigators are collaborating. Also note that each of the top-funded organizations and PIs has a history of major accomplishment in OCT, dating back to the early 1990s in some cases. As a result, they have recorded significant scientific achievement, and built strong research groups, infrastructure, and credibility with the funding sources—which results in a deserved large fraction of the funding pie. It is also important to point out that the picture has changed more recently (e.g., 2010 only) where the funding allocations are quite different across various organizations and PIs.
For NIH funding, the top 10 institutions take about 50% and the top 25 recipients have taken about 75% of the past decade’s $336M: Massachusetts General Hospital, University of Southern California, and University of California at Irvine have received the most, with $28M, $24M, and $18M, respectively. For NSF funding, the Massachusetts Institute of Technology, University of Illinois at Urbana-Champaign, and Duke University represent the top three recipients with just over $1M each. As for individuals, D. Huang, L. Wang, R. Varma, M. Ip, G. Tearney, M. Brezinski, J. Werner, J. Fujimoto, S. Boppart, and B. Tromberg are the top 10 NIH OCT funding recipients (listed as PIs on the grants); each has received more than $5M. S. Boppart, J. Fujimoto, and J. Izatt are the top three NSF OCT Fund recipients, with each receiving about $1M. The top UK funding recipients are R. Tatam, R. Hogg, A. Podoleanu, R. Wang, H. Coles, S. Matcher, R. Smallwood, and H. Liang; they have received >$1M each. Note that some of these EPSRC/BBSRC/MRC programs are for generic technology development (not mainly OCT), but in keeping with the original search criterion, they were included. The top Canadian fund recipients—A. Vitkin, V. Yang, M. Saurnic, K. Bizheva, T. Tiedje, and Brian Wilson—received in excess of $0.5M.
Outcomes in ophthalmology and beyond
As with any research, it is difficult to precisely determine the impact of taxpayer investment in OCT. However, it is clear that it was instrumental in advancing the development of OCT and its application in ophthalmology. For instance, Carl Zeiss Meditec’s highly successful OCT product line can be traced back to a startup company (Advanced Ophthalmic Devices) that came out of academic sponsored research at MIT. OCT was voted as one of the most important advances in ophthalmology over the past 25 years because it has helped diagnose various eye conditions for millions of people worldwide. A high-resolution, general-purpose diagnostic tool, OCT has played an important role in many areas of ophthalmology. For instance, OCT is the clinical standard for assessing response to treatment (using intravitreal injection of anti-angiogenesis medications) of age-related macular degeneration (AMD). The approach is a major breakthrough, and OCT has enabled it to save the sight of people who would otherwise have gone blind.
OCT is also playing major roles in the management of other macular diseases, glaucoma, and corneal disease. Importantly, it enables non-specialists—such as comprehensive ophthalmologists or optometrists—to detect early disease at a level approaching that of retinal and glaucoma specialists. Early detection, at stages where disease is still treatable, has had a dramatic impact on patient care.
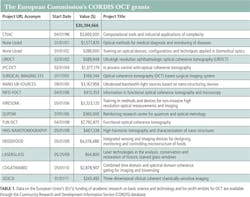
Was the NEI NIH budget of approximately $131M over the past decade a good investment? (See Table 1.) From both healthcare and business return-on-investment (over $1B in revenue generated) perspectives, most people would say yes. Current and future funding may be equally important to understand the progression of diseases and to sponsor high-risk technology development for new instrumentation with advanced capabilities for treatment of blinding diseases like glaucoma and AMD.
Cardiology is another area in which OCT seems destined to have a major impact on both the medical device industry and healthcare. Cardiovascular disease is the #1 killer in the industrialized world, affecting approximately one million people annually in the U.S. Intravascular OCT may play an important role in identifying and guiding treatment, and enabling diagnosis necessary for clinical studies on technologies such as stents and drugs. Today, three companies have major intravascular OCT product efforts: LightLab Imaging/St. Jude Medical (which currently has the only FDA-approved product), Volcano Corp., and Terumo. LightLab received FDA clearance in 2010 and shortly afterward was acquired by St. Jude Medical for approximately $90M. Already, more than 40,000 patients have been imaged with LightLab clinical instruments. While none of these companies has received significant direct U.S. government funding, all can be clearly traced to government-sponsored research in academe: LightLab to MIT, the Volcano OCT product to the University of Texas, and Terumo’s to research at Massachusetts General Hospital. As with ophthalmology, it is probably equally important that the government continue sponsoring work in this area.
OCT is poised to make important scientific contributions in dermatology, pulmonology, otolaryngology, gastroenterology, gynecology, developmental biology, and dentistry, too. As the technology begins to make major impact beyond ophthalmology, and is further integrated with other diagnostic imaging modalities and therapeutic procedures, it will enable still more fundamental scientific and clinical advances, and pay back handsomely in terms of for-profit company and jobs creation. In Part Two of this article, we’ll take a look at for-profit companies that have benefitted from government grants—directly and indirectly. Find Part Two in advance of print at https://www.laserfocusworld.com/14190292.
ACKNOWLEDGEMENTS
The author thanks the following people for help retrieving data from databases: Russell Cox (EPSRC); Nancy Mendoza (BBRSC); the CORDIS Support Service (EC CORDIS); and Tiffany Lay (MRC)—as well as for permission to reproduce the data; also Yvonne Mason from AFOSR for funding data on the Medical Free Electron Laser Program; and James Fujimoto at MIT for reviewing and discussing this market research. Exchange rates used in this article are: U.S. $1.64/£ , $1.44/€, and $1.025/Canadian dollar.
A pioneer in optical coherence tomography (OCT) and a serial entrepreneur, Eric A. Swanson is co-founder of such companies as Advanced Ophthalmic Devices and LightLab Imaging. Among his activities in technology research and development, he serves on various boards for companies, including NinePoint Medical. He is also editor of the non-profit news outlet, OCT News (www.octnews.org). Contact him at [email protected].