Zeiss receives FDA approval for OCT angiography technology
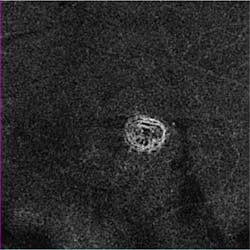
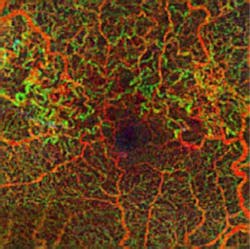
Zeiss Medical Technology (Dublin, CA) has received FDA clearance for its AngioPlex OCT angiography technology, which enables ophthalmologists to use optical coherence tomography (OCT) images to assess the blood vessels (vasculature) of the retina at a depth and clarity never available before. The technology delivers high-resolution, depth-resolved visualization of the separate layers of the retinal and choroidal vasculature without the need for an injected contrast dye, as is standard with fluorescein angiography (FA).
The information provided by these images is clinically impactful, because progression of retinal diseases is often accompanied by changes in the vasculature of the eye. In age-related macular degeneration (AMD), diabetic retinopathy, central retinal vein occlusion, and other vascular conditions, AngioPlex OCT angiography can complement traditional FA and become a safe and efficient part of routine eye care, potentially enabling earlier detection and management of micro-progressions.
AngioPlex OCT angiography clearly visualizes blood flow by detecting motion of scattering particles, such as red blood cells, within sequential OCT B-scans performed repeatedly at the same location of the retina. Unlike other OCT angiography systems that require multiple OCT scans to generate one single OCT angiography image, the company's CIRRUS HD-OCT system with AngioPlex only requires a single additional OCT scan. The key to this is the real-time retinal tracking system, FastTrac, that actively eliminates eye motion to provide motion-artifact-free images of the perfused retina. Equally important, FastTrac enables follow-up OCT angiography images to be acquired at the same precise location to assess treatment efficacy and monitor disease progression.
AngioPlex is powered by Optical Micro Angiography (OMAG) algorithms to provide ultra-clear vascular images. OMAG is an image processing technique that takes full advantage of not only amplitude, but also phase OCT signal data to deliver the highest-quality OCT angiography images.
AngioPlex OCT Angiography is available on the CIRRUS 5000 HD-OCT platform, allowing ophthalmic practices the flexibility to easily integrate vascular imaging with standard OCT diagnostic imaging.
In addition to FDA clearance of AngioPlex, CE Marking is pending from the European Union.
For more information, please visit www.zeiss.com/meditec.
Follow us on Twitter, 'like' us on Facebook, connect with us on Google+, and join our group on LinkedIn