VITAL SIGNS MONITORING: Speckle effect enables noninvasive glucose, pulse readings
In papers published by Biomedical Optics Express, groups of researchers from the Netherlands and Israel describe two new wearable devices that use changing patterns of scattered light to monitor biometrics. One is reportedly the first wearable device able to measure glucose concentration directly but noninvasively, and it also measures hydration. The second measures heart rate and has proven less sensitive than other wearable pulse monitors to errors when the wearer is moving—for instance, walking or playing sports.
Both of the watch-like devices leverage "speckle"—the grainy interference patterns that result when laser light reflects from an uneven surface or scatters from an opaque material. When the material that is scattering the light is moving—say, in the case of blood flowing through the circulatory system—"the speckle pattern changes with changes in the flow," explained biomedical engineer Mahsa Nemati, a graduate student in the Optics Research Group at the Delft University of Technology (the Netherlands) and lead author of the pulse-monitoring paper. Those light variations are a valuable source of information, she says.
Measuring glucose and hydration
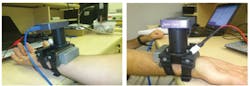
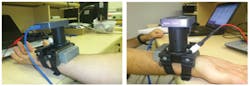
In the first paper, bioengineer Zeev Zalevsky of Bar-Ilan University (Ramat Gan, Israel) and colleagues describe how a laser generates a wavefront of light that illuminates a patch of skin on the wrist near an artery, and how a camera measures changes over time in the light that is backscattered off the skin.1 Unlike other chemicals present in the blood, glucose exhibits a so-called Faraday effect. This means that in the presence of an external magnetic field (generated by a magnet attached to the device), the glucose molecule alters the polarization of the wavefront and thus influences the resulting speckle patterns. Analyzing these changing patterns provides a direct measurement of the glucose concentration. Because one of the main signs of mild to moderate dehydration is muscle weakness, which will alter the strength of the signals, the same device can also be used to indicate the relative dehydration level of the user as it changes over time.
Zalevsky and his colleagues are now working to reduce the margin of error in the device's readings. "Around 96 percent of our in vivo measurements were within a range of 15 percent deviation from the readout of a medical reference glucometer device," Zalevsky noted. "The main factor for errors now is the stability of our device on the wrist of the user. We are currently investing efforts in deriving proper calibration and motion cancellation procedures that will allow us to reduce this sensitivity."
Zalevsky says this is a first step toward noninvasive, continuous in vivo measurement of glucose that is based on sensing an effect directly related to glucose concentration. The team expects a commercial version of the device to reach the market in 2-3 years.
Tracking heart rate
Using simulated heart beats generated in milk and measurements performed on the finger of a volunteer, Nemati and her colleagues at Delft and at Phillips Research found that speckle changes can be used to accurately measure flow pulsations—that is, the heart rate—even when the light source used to create the speckle pattern is also moving. They also found that just a couple of pixels from the image were sufficient to extract the pulse rate.
"This paper shows for the first time that a speckle pattern generated from a flowing liquid can give us the pulsation properties of the flow in spite of motion-induced artifacts," Nemati said of the publication describing the work.2 "Sophisticated optics is not necessary to implement this, so the costs for devices can be kept low. Another advantage is that the devices can be non-contact or far from the sample," she added.
The team is now working with companies to integrate their motion-friendly, pulse-monitoring technique into existing sensors for potential use clinically as well as in sports.
1. N. Ozana et al., Biomed. Opt. Exp., 5, 6, 1926–1940 (2014).
2. M. Nemati et al., Biomed. Opt. Exp., 5, 7, 2145–2156 (2014).