DENTISTRY/REGENERATIVE MEDICINE: Low-level light stirs in vivo stem cells to regenerate tissue
Researchers have demonstrated for the first time that noninvasive, low-power light therapy can prompt stem cells inside the body to reconstruct tissue.1 The research promises a broad range of clinical applications in restorative dentistry and regenerative medicine, including wound healing and bone growth. And it hints at dramatic changes for dentistry and other disciplines.
Led by Wyss Institute Core Faculty member David Mooney, Ph.D., of Harvard University (Cambridge, MA), the team used a low-power laser to trigger human dental stem cells to form dentin, the hard tissue that is similar to bone and makes up the bulk of teeth. Further, they outlined the precise molecular mechanism involved and demonstrated its abilities using multiple laboratory and animal models.
A number of biologically active molecules, such as regulatory proteins called growth factors, can cause stem cells to differentiate into different cell types. Current regeneration efforts require scientists to isolate stem cells from the body, manipulate them in a laboratory, and return them to the body—efforts that face regulatory and technical hurdles to clinical translation. Mooney hopes the new approach will be easily adopted by practicing clinicians. "Our treatment modality does not introduce anything new to the body, and lasers are routinely used in medicine and dentistry, so the barriers to clinical translation are low," said Mooney, who is also the Robert P. Pinkas Family Professor of Bioengineering at Harvard's School of Engineering and Applied Sciences (SEAS). "It would be a substantial advance in the field if we can regenerate teeth rather than replace them."
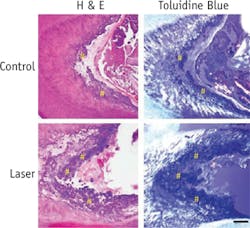
At the time of the research, Praveen Arany, DDS, Ph.D., who is now an Assistant Clinical Investigator at the National Institutes of Health (NIH), was a Harvard graduate student and then postdoctoral fellow affiliated with SEAS and the Wyss Institute. Arany, lead author of a paper describing the work, took rodents to the laboratory version of a dentist's office to drill holes in their molars, treated the tooth pulp that contains adult dental stem cells with low-dose laser therapy, applied temporary caps, and kept the animals comfortable and healthy. After about 12 weeks, high-resolution x-ray imaging and microscopy confirmed that the laser treatments had enhanced dentin formation. The dentin was strikingly similar in composition to normal dentin, but had slightly different morphological organization. Moreover, the typical reparative dentin bridge seen in human teeth was not as readily apparent in the rodent teeth, owing to technical challenges with the procedure.
Next, the team performed a series of culture-based experiments to elucidate the exact molecular mechanism responsible for the regenerative effects of the laser treatment. It turns out that a ubiquitous regulatory cell protein called transforming growth factor beta-1 (TGF-β1) played a pivotal role in triggering the dental stem cells to grow into dentin. TGF-β1 exists in latent form until activated by any number of molecules. The team confirmed a chemical domino effect: In a dose-dependent manner, the laser first induced reactive oxygen species (ROS), which are chemically active molecules containing oxygen that play an important role in cellular function. The ROS activated the latent TGF-β1 complex, which in turn differentiated the stem cells into dentin.
Understanding the mechanism was key because since the first use of medical lasers in the late 1960s, doctors have been accumulating anecdotal evidence that low-level light therapy (LLLT, also known as photobiomodulation, or PBM) can stimulate all kind of biological processes, including skin rejuvenation and hair growth. But the clinical effects of LLLT have been subtle and largely inconsistent. The new work marks the first time that scientists have gotten to the nub of how it work on a molecular level, and lays the foundation for controlled treatment protocols.
"The scientific community is actively exploring a host of approaches to using stem cells for tissue regeneration efforts," said Wyss Institute founding director Don Ingber, MD, Ph.D., "and Dave and his team have added an innovative, noninvasive, and remarkably simple but powerful tool to the toolbox."
Arany aims to take this work to human clinical trials. He is currently working with his colleagues at the National Institute of Dental and Craniofacial Research (NIDCR) to outline the requisite safety and efficacy parameters. "We are also excited about expanding these observations to other regenerative applications with other types of stem cells," he said.
1. P. R. Arany et al., Sci. Trans. Med., 6, 238, 238ra69 (2014); doi:10.1126/ scitranslmed.3008234.