LIGHT THERAPY: Low-level laser therapy: Treatment through precise application of light
RYAN MALONEY
When administered with specific output parameters, light has powerful medical benefits. In fact, light has been used for centuries to treat specific conditions. For instance, ancient Egyptians constructed solariums—rooms fitted with colored glass to harness specific colors of the visible spectrum—to treat prevalent ailments.
Over the years, the medical application of light has evolved, with newer technologies capable of more precise parameters. Greater precision allows clinicians to target specific light-absorbing molecules to treat select tissues. An application of light that epitomizes precision is low-level laser therapy (LLLT).
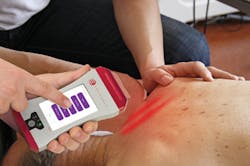
LLLT administers an exact wavelength, or color of light, in a coherent manner, meaning that photons propagate in the same direction, amplitude, and phase (see Fig. 1). Coherence is an important characteristic for maximizing the depth of light penetration. This is key, as a specific dose (or energy) of light is required to trigger a biological response. Therefore, coherence maximizes the amount of light energy penetrating the skin to reach deeper therapeutic targets.1
However, a laser dose adheres to a biphasic model, because extreme energies (too little or too great) limit cellular response.2 The old adage "more is better" does not apply to LLLT. It operates under the principles of photochemistry (the study of chemical and biochemical reactions triggered by light absorption), as opposed to photothermogenesis (wherein light is used to generate heat). In most cases, light is absorbed by biologically active structures such as enzymes or proteins. Therefore, overstimulation of phototargets may inhibit their biological function and cause untoward effects: great importance is placed on understanding the mechanism and biphasic model of LLLT, as well as the properties of the phototarget.
Tissue absorbs light
Although the exact mechanism remains elusive, studies have made tremendous progress to understand how LLLT alters cell function and, in turn, treats certain medical conditions. In order for laser therapy to modulate cellular behavior, light energy must first be absorbed. Responsible for the absorption of light are chromophores or photoacceptor molecules.3 These molecules possess a structure with a well-defined absorption spectrum, and therefore have a narrow absorption band. As these molecules absorb specific bands of light, most enter an electronically excited state and can act as a signal transducer, transmitting intracellular signals that affect cytoplasmic machinery and thereby alter cell behavior.3 Accordingly, understanding the absorption spectra and biological role of a phototarget may help predict the biological response after laser exposure.
A well-studied phototarget that acts as a signal transducer is cytochrome c oxidase (COX), an integral membrane protein positioned within the inner mitochondrial membrane. This enzyme is responsible for shuttling protons across the inner mitochondrial membrane to establish an electrochemical gradient to produce adenosine triphosphate (ATP). Laser therapy accelerates electron and proton transfer, resulting in a greater production of ATP.
ATP is an important biological catalyst, driving a majority of cell function. Laser therapy increases the synthesis of ATP, briefly increasing cytoplasmic ATP and thereby affecting the cell's intracellular machinery. This sequence of events is broken into two basic phases: the primary phase, which describes the initial upregulation of ATP synthesis; and the secondary phase, which describes what pathways were affected by the elevated ATP.3 In most cases, the phototarget is ubiquitous. Therefore, the observed clinical response of laser therapy is based on the secondary phase: how the intracellular machinery interprets or responds to the excited phototarget. This helps explain why laser therapy induces a different biological response when treating different cells that express the same phototarget. For example, exposure to a 635 nm laser causes adipocytes to expel intracellular lipids while causing fibroblasts to proliferate and synthesize collagen.4,5 Both responses theoretically stem from stimulating COX; thus, a laser administering a specific wavelength excites a phototarget that induces the cell to respond according to its predetermined state.
Applications and verifications
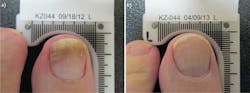
To understand how cells respond to specific wavelengths, academic and private institutions have continued to study light-based therapies. An organization that has helped pioneer LLLT by helping to validate its efficacy is Erchonia, a developer of aesthetic and medical solutions grounded in evidence-based medicine. The company has been building and studying low-level laser devices for more than a decade, and has earned eight separate U.S. Food and Drug Administration (FDA) market clearances. The initial clearance in 2002, for the treatment of acute and chronic neck and shoulder pain, was the first clearance granted in the U.S. for a low-level laser device (see Fig. 2). Each potential therapeutic application undergoes a placebo-controlled, randomized, double-blind, multicenter clinical study. Completing high-quality studies has been important because laser therapy, as a whole, remains a controversial treatment. A randomized, controlled trial design aims at eliminating confounding variables to provide the medical community with an objective evaluation of the technology.Ongoing clinical studies are now evaluating the role the MLScanner can play in the fight against obesity. Technologies like this demonstrate the progress laser therapy has made in recent years.
LLLT remains a controversial treatment, but with important objective studies being conducted to evaluate the technology, this subtle, noninvasive treatment is becoming an appealing therapeutic option. Only with sufficient demonstration of the application's clinical utility will the whole of the medical community view LLLT as a viable treatment.
REFERENCES
1. T. I. Karu, "Low power laser therapy," Biomedical Photonics Handbook, CRC Press, Boca Raton, FL (2003).
2. Y. Y. Huang, S. K. Sharma, J. Carroll, and M. R. Hamblin, Dose-Response, 9, 4, 602–618 (2011).
3. T. Karu, J. Photochem. Photobiol. B, 49, 1, 1–17 (1999).
4. R. Neira et al., Plast. Reconstr. Surg., 110, 3, 912–922 (2002).
5. D. Hawkins and H. Abrahamse, Photomed. Laser Surg., 25, 3, 159–169 (2007).
6. C. Roehlecke et al., PLoS One, 8, 9, e71570 (2013).
7. M. Schindl et al., Photodermatol. Photoimmunol. Photomed., 15, 1, 18–21 (Feb. 1999).
8. A. Schindl et al., J. Am. Acad. Dermatol., 40, 3, 481–484 (Mar. 1999).
9. W. B. Lim et al., Lasers Surg. Med., 43, 4, 344–352 (2011).
10. R. Duan, T. C. Liu, Y. Li, N. Guo, and L. Yao, Lasers Surg. Med., 29, 2, 174–178 (2001).
Ryan Maloney is a research consultant for Erchonia Medical, McKinney, TX; e-mail: [email protected]; www.erchonia.com.