An international team of researchers, including Lawrence Livermore National Laboratory (LLNL; Livermore, CA) physicist Matthias Frank and postdoctoral researcher Mark Hunter, have for the first time used an ultra-intense x-ray laser to determine the previously unknown atomic-scale structure of a protein.
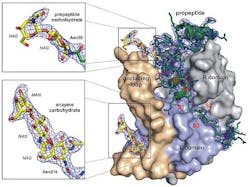
The team determined the structure of an enzyme key to the survival of the single-celled parasite Trypanosoma brucei, responsible for African sleeping sickness, a disease that kills 30,000 people each year.
This new structural information should help guide the search for drugs that act like the propeptide, tying up the enzyme and killing the parasite. To determine the structure of the precursor form of the protein--which does not form crystals large enough for traditional x-ray diffraction--submicron nanocrystals produced by the parasite were analyzed by the "diffraction before destruction" technique, in which individual nanocrystals are passed, one by one, through the x-ray beam at the Linac Coherent Light Source (LCLS), followed by "stacking" of the resultant diffraction data (in this case, from 178,875 individual nanocrystals).
The achievement also demonstrates that the approach can provide otherwise unobtainable biomolecular information, potentially ushering in a new era of protein crystallography.
The Livermore researchers--whose participation in the research is supported by the LDRD Program--developed the nanoparticle injectors, set up the laser pump probe experiments, prepared the samples, modeled the damage, and acquired data at the LCLS.
Full details of the work appear in Science; for more information, please visit http://www.sciencemag.org/content/339/6116/227.abstract?sid=6c1a9120-9e83-4359-87b0-f22a20de08d2.
-----
Follow us on Twitter, 'like' us on Facebook, and join our group on LinkedIn
Subscribe now to BioOptics World magazine; it's free!