First-ever light-operated drug hones on most common target proteins
An international team of researchers has developed the first-ever light-controlled therapeutic agent whose effects focus specifically on the largest, most important class of drug target proteins—G protein-coupled receptors (GPCRs). The ability to control drug activity with light means that the therapeutic effects can be accurately delivered locally, thus reducing their effect on other areas and the resultant side effects, and helping to reduce the dosage required.
Related: Photo-switchable peptides promise precision control of drugs, and limited side effects
The research team was led by scientists in the Nanoprobes and Nanoswitches group of the Institute for Bioengineering of Catalonia (IBEC; Barcelona, Spain), and involved colleagues from the Institute of Advanced Chemistry of Catalonia (IQAC-CSIC), the Autonomous University of Barcelona (UAB), and the Bellvitge Biomedical Research Institute (IDIBELL), all in Barcelona, and the Institute for Functional Genomics (Montpellier, France).
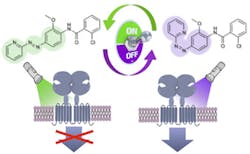
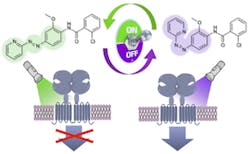
The scientists developed Alloswitch-1, a small-molecule therapeutic agent that modulates drug target protein receptors specifically at the allosteric, or non-active, site of the protein. Allosteric modulators have a number of advantages over traditional drugs, offering higher selectivity of target receptors, tunable release according to whether the undesirable protein receptor activity is present, and lower potential for toxic effects.
“To build a photocontrolled modulator of a G protein-coupled receptor, we had to develop a new chemical design concept in which the photoswitch is not tethered to the drug but inserted within the pharmacophore, which is the group of atoms in the molecule of a drug responsible for its action,” explains Pau Gorostiza, group leader of IBEC’s Nanoprobes and Nanoswitches group. “We also got a bit lucky, because we not only obtained a photoswitchable GPCR ligand, but one of the most potent and selective allosteric modulators in its class.”
The effects of this ‘optopharmacological’ compound can be remotely controlled in space and time in living, wild-type organisms. This is an advantage over optogenetic manipulations, which require gene overexpression using viruses, for example.
Small molecule therapeutic agents such as Alloswitch-1, if they can be made available orally, could offer a competitive advantage over traditional drugs, which often affect off-target tissues and organs, leading to unwanted consequences and compromising their beneficial effects.
Full details of the work appear in the journal Nature Chemical Biology; for more information, please visit http://dx.doi.org/10.1038/nchembio.1612.
-----
Follow us on Twitter, 'like' us on Facebook, connect with us on Google+, and join our group on LinkedIn
Subscribe now to BioOptics World magazine; it's free!