Raman makes flow cytometry flexible
The addition of spectroscopy to flow cytometry is allowing researchers to characterize samples with unidentified autofluorescence. In addition, spectroscopic methods–especially Raman–are broadening the number of variables that can be observed simultaneously.
By GERALD CAIRNS
Spectral flow cytometry–the addition of spectroscopic methods to traditional cell analysis technology–promises to significantly expand the usefulness of flow cytometers for cell biology and molecular analysis (see bioopticsworld.com/articles/359253).
Traditional flow cytometers identify cells labeled with fluorescent dyes by suspending them in fluid and streaming them past a set of laser beams (see bioopticsworld.com/articles/355173). The scattered and fluorescent light from the cells passes through wavelength-selective filters and dichroic mirrors to single point detectors–typically photomultiplier tubes (PMTs) or avalanche photo diodes (APDs). The intensity and nature of the signal is then used to determine the physical and chemical properties of the cells.
To read the full version of this article please click here to login
But as powerful as it is, conventional cytometry has some fundamental limitations. For one, the spectra of the fluorescent dyes used must be known–and researchers studying naturally occurring cell autofluorescence typically don’t know the correlating spectra.
Also, while use of a greater number of fluorescent labels enables identification of a greater range of cell types, today’s state-of-the-art instruments make it possible to identify only 19 parameters simultaneously. The wavelength range at which each label emits is quite broad–so when more multiple labels are used, the dyes’ emission spectra overlap until the fluorescent signals become undistinguishable from each other.1
Overcoming limitations
Spectral flow cytometry (SFC) elegantly addresses the first limitation–the issue of knowing dye spectra–by directly recording the full fluorescence spectra of individual cells, allowing characterization of samples with unknown intrinsic fluorescence properties. This advance is made possible by replacing the filters and wavelength-selective mirrors used in traditional single-point detector-based flow cytometry with a spectrograph attached to a single CCD array detector. Called an electron-multiplying charge-coupled device (EMCCD), this ultrasensitive and fast scientific spectroscopy camera was first introduced by Andor Technology (Belfast, Northern Ireland) in 2005.
A spectral flow cytometer developed using an Andor Newton EMCCD camera (Newton DU970N)–which has a fast read-out rate and short exposure time compared to traditional charge-coupled-device (CCD) cameras and is more sensitive than PMTs, allowing a better signal-to-noise ratio–opens up the possibility of using the intrinsic autofluorescence properties of cells for diagnostic purposes.2 Preliminary studies are seeking to use cellular autofluorescence to differentiate among cultured cell lines.3
As for the limit in the number of distinct labels that can be used to stain cells, one possible way to overcome this limit is to use labels beyond the traditional visible spectrum. However, this approach requires the integration of more light sources, detectors, and optics, leading to an associated growth in the complexity of the flow-cytometer hardware and analysis software. Expense and difficulty of operation would also increase. An alternative approach is to use Raman scattering as a complement to fluorescence labels.
Raman: oodles of info
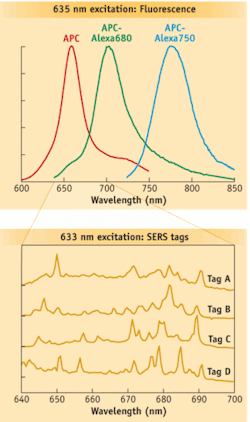
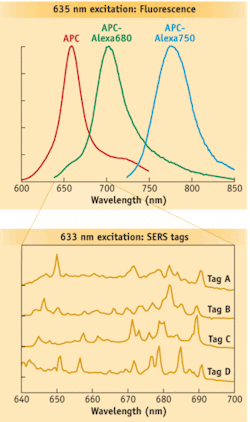
Raman spectral flow cytometry is a potential complement to current techniques and has the potential to increase significantly the number of distinct labels available to identify cells. It relies on Raman scattering, which occurs when light–typically from a laser–is reflected in a way that is linked to the nature of the chemical bonds of the molecules analyzed. Raman spectra (“fingerprints”) have features that are much narrower than fluorescence spectra (see Fig. 1), providing the potential to convey much more information. Mathematical spectral unmixing approaches allow the fingerprints of multiple tags to be distinguished.
The challenge to the successful adaptation of Raman spectroscopy to flow cytometry is to make the measurement during the very short time each cell is in the laser beam.
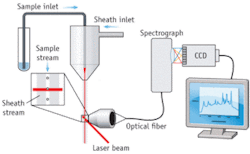
Scientists at La Jolla Bioengineering Institute (LJBI; La Jolla, CA) and Los Alamos National Laboratory (Los Alamos, NM) recently made the first successful measurements of Raman spectra from individual particles in flow.4 Their custom, proof-of-concept instrument used Rayleigh light scatter to trigger the EMCCD to capture Raman spectra dispersed by an imaging spectrograph (see Fig. 2). Nanoparticles composed of silver-core-bearing Raman scattering labels and coated in glass were attached to biomolecules, such as antibodies. These nanoparticles exhibit surface-enhanced Raman scattering (SERS), a phenomenon that greatly increases the brightness of the Raman scattering and helps make feasible rapid measurements in flow. The initial demonstration used polystyrene beads as a surrogate for cells, and enabled differentiation among four different SERS tags.
More recently, the LaJolla and Los Alamos researchers collaborated with scientists at LJBI to adapt a commercial flow cytometer, a Union Biometrica COPAS Plus, to collect Raman spectra.5 The high-speed spectral analysis of individual particles in flow will enable new applications in biological and chemical analyses, the researchers report. According to Prof. John Nolan of LJBI, “All of this work is really at the proof-of-principle and validation stage. I would say it would be another year before we see rigorous and compelling biological demonstrations in the peer-reviewed literature.”
Pending such demonstrations, though, spectroscopic flow cytometry could find a crucial role in research and medicine. The introduction of Raman scattering as a flow-cytometry parameter will significantly increase the already formidable power of flow cytometry for highly multiparameter and multiplexed applications. And the collection of full spectral information from individual cells at high speeds may enable new approaches to disease, diagnostics, and treatment–for diagnosing cancer, identifying cancer stem cells, and monitoring treatment, for example. It is expected that continued development of CCD detector technology will translate to even further improvements in speed, sensitivity, and resolution.
REFERENCES
- P.K. Chattopadhyay et al., Immunology 125, p. 441 (2008).
- G.R. Goddard et al., Cytometry 69A, p. 842 (2006).
- G.R. Goddard et al., Proc. of SPIE. 6859, p. 1 (2008).
- D.A. Watson, et al., Cytometry Part A 73A, p. 119 (2008).
- D.A. Watson et al., Cytometry Part A 75, p. 460 (2009).
GERALD CAIRNS is application specialist for spectroscopy at Andor Technology, Belfast, Northern Ireland; www.andor.com; e-mail: [email protected].