Research team resolves true structure of KR2 rhodopsin protein for optogenetics
A team of researchers from the Moscow Institute of Physics and Technology (MIPT; Moscow, Russia) and colleagues has discovered and studied the structure of the KR2 rhodopsin under physiological conditions, which could lead to a new optogenetic instrument for efficient therapy of depression, anxiety disorders, epilepsy, and Parkinson's disease.
Optogenetics investigates techniques for controlling the nerve and muscle cells in a living organism via light signals. Optogenetic methods already enable a partial recovery of lost eyesight, hearing, and muscle control impaired by a neurological disease. Importantly, these techniques allow researchers to study neural networks in detail. This refers not to computer networks, but to those housed in the human brain and responsible for our emotions, decision-making, and other fundamental processes.
Several years ago, researchers discovered a new type of ion transporter—the KR2 rhodopsin—in the cell membrane of the marine bacterium Krokinobacter eikastus. The newly found protein is sensitive to light, making it useful for optogenetics. Driven by light, such proteins can facilitate the translocation of charged particles such as ions across the cell membrane. By introducing such transporters into the cell, researchers can then use light pulses to manipulate the potential of the neuron cell membrane, controlling its activity. KR2 was shown to selectively transport a particular kind of particles—sodium ions—outside the cell. Rather than allow the passage of these ions in both directions, the protein performs active transport, serving as a "pump." Mutant forms of KR2 also showed potassium-pumping activity. By implanting these pumps into the cell membrane, the whole scope of neuron activity could theoretically be controlled.
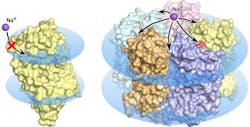
The wave of research that followed the discovery of the new molecular pump faced some pretty mysterious properties of the rhodopsin. Several research groups discovered and described a total of five different structures of the promising protein. Notably, in some of these structures five KR2 molecules form a stable pentamer, while in others only the protein monomer is present.
Led by MIPT biophysicists, the research team found what gives rise to the confusing variety of protein structures. It turned out that the research groups studying KR2 had crystallized the protein at different conditions. The unique protein is originally produced by an ocean bacterium native to a very special environment. It lives in water with a specific salinity, acidity, and hydrogen ion concentration (pH). These conditions are a prerequisite for the protein to do what researchers expect it to do—that is, pump sodium ions, while also forming pentamers in the cell membrane. The protein's numerous "false" structures turned out to either be crystallization artifacts or only correspond to the conditions that virtually disable the sodium-pumping activity of KR2, which makes it highly attractive for the global optogenetics community.
"...we have simulated the physiological conditions for KR2 existence and functioning. As a result, we obtained the 'correct' structure of the new protein, which corresponds to its native state. We showed that the functional unit of the protein is a pentamer," explains Valentin Gordeliy from the Institute of Structural Biology (Grenoble, France), an author of the study. "On top of that, we found an explanation for the contradictions between previous structural studies of the protein."
The KR2 rhodopsin is revolutionary for optogenetics, and knowing its correct structure under physiological conditions is fundamental both for understanding the mechanisms behind its functioning and for exploring the nervous system by modeling new optogenetic tools and applying them in the medical practice.
Full details of the work appear in the journal Science Advances.