MULTISPECTRAL IMAGING/NEUROSCIENCE: Multispectral system enables brain development discovery
Multispectral imaging has enabled the discovery that blood flow is controlled differently by infant and adult brains.1 The researchers at Columbia Engineering (New York, NY) who uncovered this fact say it could change the way brain development is studied.
Elizabeth Hillman, associate professor of biomedical engineering and radiology, led the study in her Laboratory for Functional Optical Imaging. She said that the control of blood flow in the brain is very important: Regionally, specific increases in blood flow are necessary for normal brain function, and they generate signals measured by functional magnetic resonance imaging (fMRI), a widely used assessment tool. According to Mariel Kozberg, a neurobiology MD-PhD candidate who is lead author of the paper, the team tracked changes in control with increasing age. In doing so, they observed that the brain gradually develops its ability to increase local flow, and by adulthood, generates a large response to stimuli.
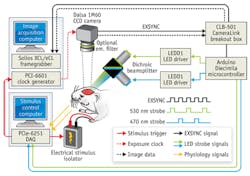
The team used a multispectral optical intrinsic signal imaging (MS-OISI) system, previously developed in the same lab, to conduct the research (see figure).2 MS-OISI is a high-speed, high-resolution approach that uses the different absorption spectra of deoxygenated and oxygenated hemoglobin to determine changes in the concentrations of each. "Our results suggest that the infant brain might not be able to generate localized blood-flow increases, even if there is neuronal activity occurring, and that the development of blood-flow control occurs in parallel with early neuronal development," said Kozberg. "This could suggest that fMRI studies of infants and children may be detecting changes in both vascular and neuronal development-in fact, vascular development may be an important new factor to consider in normal and abnormal brain development."
The team also found that the younger age groups were highly sensitive to blood pressure increases in response to stimulation and that these increases can cause large increases in blood flow across the brain. "This finding indicates that the newborn brain is also unable to regulate its overall blood-flow levels," Kozberg explains. "This could explain earlier fMRI results in infants and children that were sometimes positive and sometimes negative, because it is difficult to tell whether blood pressure increases are occurring in infants and children. This result suggests that great care should be taken in setting stimulus thresholds in young subjects."
The researchers add that, since the newborn brain appears to be able to sustain itself without tightly controlled blood flow, their findings suggest that the infant brain may be intrinsically more resistant to damage due to a lack of oxygen than the adult brain. "This could be an important property to understand, both in terms of understanding how best to treat blood-flow problems in the newborn infant brain, which can cause lifelong problems such as cerebral palsy, and to potentially better understand how to treat the adult brain in conditions such as stroke," Hillman said.
Next steps include further defining the cellular mechanisms underlying the developing hemodynamic response at a cellular and microvascular level, using methods such as high-speed and multi-plane in vivo two-photon microscopy, another technique developed in the lab. They're particularly interested in tracking changes in neuronal activity, microvascular architecture and connectivity, and the distribution and activity of other cellular populations thought to be associated with neurovascular coupling as a function of development. Hillman adds that her team is also looking to take this research into the clinic and explore whether their findings could improve diagnosis and monitoring of newborn infants.
Incidentally, an optimized version of the MS-OISI system, implemented with an inexpensive FireWire camera, is described in a separate paper.3 The authors made companion software widely available online so that others can replicate the system.
1. M. Kozberg, B. Chen, S. E. DeLeo, M. Bouchard, and E. Hillman, Proc. Nat. Acad. Sci., doi:10.1073/pnas.1212785110 (2013).
2. M. B. Bouchard, B. R. Chen, S. A. Burgess, and E. M. C. Hillman, Opt. Exp., 17, 18, 15670–15678 (2009).
3. R. Sun, M. B. Bouchard, and E. M. C. Hillman, Biomed. Opt. Exp., 1, 2, 385–397 (2010).