Spectroscopy method clarifies molecular process of anti-HIV drug
A team of researchers from the University of Chicago (UChicago; Illinois) and the Massachusetts Institute of Technology (MIT; Cambridge, MA) used a spectroscopy method to help clarify the poorly understood molecular process by which an anti-HIV drug induces lethal mutations in the virus' genetic material. The findings could bolster efforts to develop the next generation of anti-viral treatments.
Related: Spectroscopy techniques assist in revealing how potential HIV drug destroys the virus
Viruses can mutate rapidly to adapt to environmental pressure. This feature also helps them become resistant to anti-viral drugs. But scientists have developed therapeutic anti-viral agents for HIV, hepatitis C, and influenza using a strategy called lethal mutagenesis.
This strategy seeks to extinguish viruses by forcing their already high mutation rates above an intolerable threshold. If viruses experience too many mutations, they can't properly manage their genetic material.
"They can't replicate and so are quickly eliminated," says Andrei Tokmakoff, the Henry G. Gale Distinguished Service Professor in Chemistry at UChicago. "In order to make this work, you need a stealth mutagen. You need something sneaky, something that the virus isn't going to recognize as a problem."
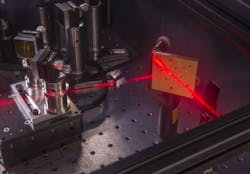
Tokmakoff and his associates at UChicago and MIT worked with the anti-HIV agent KP1212, collecting data with two-dimensional infrared (IR) spectroscopy, which combines ultrafast time resolution with high sensitivity to chemical structure.
"Two-dimensional infrared spectroscopy will be critical on the path ahead. It lets us look at the structures that exist in aqueous solution, which is the natural milieu of cells," says study co-author John Essigmann, MIT's William and Betsy Leitch Professor of Chemistry, Toxicology, and Biological Engineering. Essigmann is co-founder of a pharmaceutical company that is developing mutagenic inhibitors of HIV.
Scientists design lethally mutagenic molecules such as KP1212 to resemble natural DNA bases, the adenine-thymine, cytosine-guanine base pairs. "These analogs can bind to the wrong base partners and therefore lead to genetic mutations," says the study's lead author Sam Peng, who was a visiting graduate research assistant at UChicago.
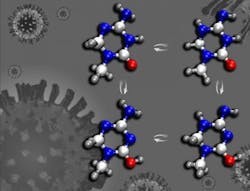
KP1212 is a cytosine variation, which normally would pair with guanine during replication. But biochemical experiments and clinical trials have shown that KP1212 induces mutations by pairing with adenine. A leading proposal suggested that KP1212 derived its mutagenicity by shapeshifting—converting into a different molecular structure by repositioning its hydrogen atoms on nitrogen and oxygen atoms.
Scientists call this shapeshifted structure a tautomer. "The shuffled hydrogen positions in rare tautomers alter the hydrogen bonding patterns, resulting in incorrect base pairing," says Peng, who completed his doctorate at MIT in 2014 and will become a postdoctoral scientist at Stanford University later this year.
Most experimental tools would have difficulty distinguishing between the normal and shapeshifted structures because they interconvert very rapidly. With two-dimensional IR spectroscopy, the UChicago team was able to distinguish between the two structures. The team also was able to measure how rapidly the shapeshifting occurs under physiological conditions: in 20 billionths of a second.
The research team expected to find only two dominant tautomers, but their experiments showed that many more exist. In addition to taking on different forms as a neutral molecule, KP1212 also could accept an extra proton, giving it a positive charge at physiological levels of acidity—pH of approximately five and a half to seven—that made possible even more rearrangements and tautomer structures.
The experiments also showed that both the protonated and non-protonated forms facilitated the viral mutation rate. Even in the absence of the protonated form, the virus still mutated, just at a lower rate.
"We found that under physiological pHs, KP1212 is significantly protonated and this protonated form induces even higher mutation rates, reaching approximately 50 percent," Peng says.
The finding that the molecule could become protonated both surprised and delighted Essigmann. The work taught his team how to create even more potent shapeshifters—by decorating the KP1212 scaffold with groups of atoms and molecules, that further raises their ability to capture protons.
“KP1212 is about 20 percent of the way toward being an ideal therapeutic mutagen. The hints given to us by the spectroscopy guide us toward even better mutagenic molecules,” Essigmann says.
Although Essigmann and Tokmakoff have known each other for years, they pursued seemingly far-removed research specialties until now. Tokmakoff’s biological research involves proteins, not DNA. But together, their research teams were able to fruitfully undertake one of the first two-dimensional IR spectroscopic studies of the therapeutic mechanism of an anti-viral drug.
Full details of the work appear in the Proceedings of the National Academy of Sciences (PNAS); for more information, please visit http://dx.doi.org/10.1073/pnas.1415974112.
-----
Follow us on Twitter, 'like' us on Facebook, connect with us on Google+, and join our group on LinkedIn