Dual-laser mass spectrometry method could enable more-precise cell study
Seeking to improve upon mass spectrometry to enhance study of the chemical composition of cells, a team of researchers in the Institute of Hygiene at the University of Münster (Münster, Germany) has developed a method to improve the spatial resolution of matrix-assisted laser desorption/ionization (MALDI) mass spectrometry by approximately one-thousandth of a millimeter.
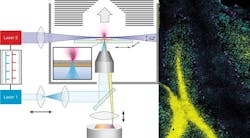
The technology, which the researchers call t-MALDI-2 (with 't' standing for transmission mode), uses two lasers: one of them generates a particularly small focus on the material removed, while the other produces the necessary signal enhancement for many biomolecules by up to several magnitudes—for example, for fat-soluble vitamins such as vitamin D, cholesterol, or administered medication. Information on their precise distribution in cells and tissues can, among other things, help to produce a better understanding of disease and inflammation processes and show new strategies for treating them.
MALDI mass spectrometry methods define the nature and the composition of molecules on the basis of their characteristic mass—that is, of their "molecular weight." This makes it possible to take a sample irradiated by the laser—for example, a thin section of tissue obtained from a biopsy—and simultaneously define often dozens, even hundreds, of different biomolecules in one measurement. However, until now, the resolution provided by mass spectrometry imaging was well below that of classic optical microscopy. With the introduction of the new t-MALDI-2 technology, however, it has been possible to noticeably reduce this gap.
"The decisive improvement which our method offers, in comparison with established MALDI imaging methods, is based on the combination and extension of two technical methods previously in use," explains Dr. Marcel Niehaus, one of the two lead authors of the study. "For one thing, in the transmission geometry, we irradiate our samples on the reverse side. This enables us to place high-quality microscope lenses very close to the sample, thus reducing the size of the laser dot. This is different from what is possible, for geometrical reasons, in standard methods—where the samples are irradiated from the direction of the mass analyzer."
However, in the minute areas of the sample that are removed by the laser, there is only an extremely small amount of material available for the subsequent mass spectrometry measurement. Therefore, the second decisive step was the use of a method called MALDI-2, which the researchers had already introduced in 2015 in the journal Science. The effect is that the so-called post-ionization laser produces an increased transfer of the initially uncharged molecules to an ionic form. If the molecules have a positive or negative charge, they are visible for the mass analyzer.In their latest study, the researchers demonstrate the possibilities offered by their technology, taking the fine structures in the cerebellum of a mouse and using kidney cell cultures. "Our method could improve the future understanding of many processes in the body at molecular level," says Klaus Dreisewerd, a professor in the Institute of Hygiene and one of the leaders of the study, along with Jens Soltwisch. "Also, established methods from optical microscopy—for example, fluorescence microscopy—could be merged with mass spectrometry imaging in a 'multimodal' instrument," Dreisewerd adds.
Full details of the work appear in the journal Nature Methods.