EARLY CANCER DETECTION/SUBCELLULAR OPTICAL IMAGING: Deep-tissue dysplasia detection with real-time subcellular analysis
ByPerry A. Genova
Angle-resolved low coherence interferometry (a/LCI) directly measures diagnostically relevant sub-cellular features in epithelial tissues up to 500 μm below the surface. Unlike optical coherence tomography (OCT), which requires image interpretation, a/LCI performs analysis of tissue and delivers to the physician direct confirmation of precancerous disease.
Because it offers the greatest opportunity for successful intervention and therapy, a key weapon in the fight against cancer is early detection. If disease can be found in the precancerous stage, before abnormal cells have had the chance to spread throughout the body, the efficacy of treatment can be quite high. The clinical challenge, therefore, is to create a method that is easy to use, widely applicable, and cost-effective.
Direct, real-time analysis
Advanced biophotonics techniques, which use light to search for disease, promise an effective means for finding precancerous lesions in the clinic. Angle-resolved low coherence interferometry (a/LCI) technology is unique in being able to evaluate microstructure in epithelial tissues up to 500 μm below the surface.1 The technique uses real-time analysis of light scattered from the tissue to detect enlargement of nuclei and other organelle-related changes indicative of early cancer progression.
This is an alternative to optical coherence tomography (OCT), which aims to construct a high-resolution image that is analyzed either by a trained expert or computer algorithm to provide diagnostic information. Instead, a/LCI seeks to measure the diagnostically relevant information directly and provide it to the physician to enable faster, more direct confirmation of precancerous disease. To date, the technique has been shown to accurately detect pre-cancer in the esophagus, colon, and cervix. Oncoscope (Durham, NC) is now developing a/LCI commercially (see sidebar, "The a/LCI system setup") to guide biopsy during upper endoscopy procedures in patients with Barrett's esophagus.
A dire need
Barrett's esophagus (BE) is an example of a clinical problem where improved detection of precancerous cells is needed and a/LCI can be effectively leveraged. BE is a metaplastic change of the esophagus associated with an increased risk of esophageal adenocarcinoma (EA).2 The survival rate of EA is abysmal; less than 15 percent of patients survive five years or more.3
Unfortunately, incidence of both BE and EA is rising sharply in the U.S., highlighting the need for improved detection of precancer. Due to the increased risk of developing cancer, BE patients are subjected to periodic surveillance, consisting of upper endoscopy with multiple biopsies. The surveillance regimen is demanding, as it requires four quadrant biopsies to be taken for every 1–2 cm of affected tissue, resulting in 20–30 biopsies per patient. Even with this degree of care, only a small amount of the tissue can be sampled-typically less than 5 percent. Other factors further lessen the effectiveness of this surveillance. These include the fact that few endoscopists rigorously follow the surveillance protocol and the documented variability in interpretation of histological biopsy specimens.
Monitoring methods
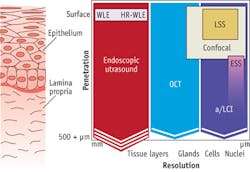
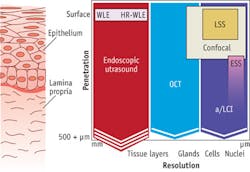
Given the acute clinical need, several techniques have been developed for monitoring BE patients (see Fig. 1). Traditional white-light endoscopy can visualize the surface of the esophageal epithelium, but its limited penetration depth means that only advanced disease can be detected, leaving open the possibility that cancer may have spread. In contrast, esophageal ultrasound can penetrate through the epithelium, but lacks the resolution to observe cellular detail.
To fill the gap between these techniques, several advanced biophotonics approaches have been applied. OCT enables three-dimensional (3D) imaging and full penetration of the epithelial layer. However, clinical implementation of OCT lacks the ability to resolve cellular detail, relegating the approach to detecting precancerous cells only in later stages, after the architecture of histological layers has been distorted.
Optical techniques that can discern cellular features, such as enlargement of cell nuclei (an important biomarker of precancerous change), include confocal microscopy and scattering spectroscopy. Confocal microscopy can visualize cell nuclei, but the limited penetration depth and sharp learning curve make it effective only in expert hands. And while spectroscopic techniques can detect enlarged nuclei, not all implementations can probe the full thickness of the epithelium in order to find precancerous cells at the earliest stages.
Just right
Able to address the limitations of these other approaches, a/LCI can measure sub-cellular features and examine the full thickness of the epithelium. Appropriate for clinical application, it has the potential to significantly improve the way precancerous tissues are monitored.
The initial indication for a/LCI use is to guide excisional biopsy during upper endoscopy Barrett's procedures by locating suspicious tissue for biopsy and histological evaluation. FDA approval for this application of Oncoscope's system in the U.S. is expected in 2016. However, additional applications to organs such as colon, cervix, and trachea have also been evaluated.
Oncoscope's a/LCI system has been used in a number of preclinical and in-man studies. In studies involving scanning animal model and ex vivo human tissues for the presence of dysplasia, a/LCI has shown outstanding performance characteristics with sensitivity as high as 100 percent and corresponding specificity as high as 90 percent. In-man trials involving more than 200 patients from two clinical centers produced a rich dataset and provided further convincing evidence that this technique is robust, sensitive, and specific. Early published results included 46 patients and demonstrated 100 percent sensitivity and 84 percent specificity.4 A fully automated prospective algorithm developed through these studies can now classify a particular tissue site as dysplastic or non-dysplastic in real time, without interpretation, and with sensitivity and specificity of 97 percent and 74 percent, respectively. The current operating characteristics strongly suggest physicians can increase the temporal and financial efficiency of these screening procedures while improving diagnostic accuracy.
The current a/LCI system is designed for use by gastroenterologists, so the probe resembles the look, feel, and operation of typical biopsy forceps. Its speed, accuracy, and simplicity are also important virtues in increasingly hectic GI endoscopy centers. Accordingly, the system provides immediate results that do not require interpretation to assist in the selection of biopsy sites. In a typical endoscopy screening procedure, the a/LCI probe is introduced through the instrument channel of a standard endoscope. The physician interrogates a specific tissue location by pressing a foot switch or a virtual button on the touch-screen, and is provided with immediate results indicating either that the scan is not suspicious for dysplasia or that a biopsy is recommended (see Fig. 2, in sidebar). This process can be quickly performed throughout the Barrett's segment and if used effectively, can reduce the total number of biopsies and procedure time while improving diagnostic yield.
Easily applied, widely applicable
Endoscopy with random biopsy is an inefficient, costly, and inaccurate method for identifying potentially precancerous tissue. This technique can sample only a small portion of the potentially affected tissue, leaving significant areas unexamined. Of those biopsies taken, only a small percentage is positive for dysplasia. Further, current screening practice involves sedation and oral endoscopy—factors that deter patients from undergoing the procedure and thereby expose them to unnecessary risk.
Oncoscope's a/LCI probe for trans-nasal endoscopy leverages the efficiency of a trans-nasal procedure, providing the full benefit of a/LCI-guided biopsy selection while increasing procedure efficiency and accuracy and reducing procedure time and cost. Such a procedure can be performed during an office visit without the complexity of a traditional endoscopy suite, and represents the next frontier of Barrett's surveillance by minimizing the impact on the patient's daily routine and by increasing procedural efficiency.
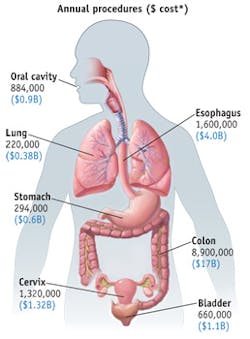
The nature of precancerous disease in the epithelium is not unique to esophageal tissue, but in fact is unilaterally applicable to all epithelial tissue types, where 85 percent of all cancers originate (see Fig. 5). Procedures employed for screening for precancerous tissue in these areas are burdened with the same challenges as those observed in esophageal endoscopy. With slight modification, Oncoscope's a/LCI system can deliver significant benefit to surveillance procedures in these other tissue targets. Accordingly, a/LCI has been successfully employed in studies involving ex vivo human cervical and colon tissues. The results from these studies are remarkably consistent with those seen in esophageal studies, clearly supporting the premise that this technology is a platform capable of transforming cancer screening procedures in many tissue targets.
The future of optical cancer screening is bright indeed.
References
1. A. Wax et al., Cancer Res., 63, 3556–3559 (2003).
2. N. Shaheen and D. F. Ransohoff, JAMA, 287, 1972–1981 (2002).
3. M. A. Eloubeidi, A. C. Mason, R. A. Desmond, and H. B. El-Serag, Am. J. Gastroenterol., 98, 1627–1633 (2003).
4. A. Wax, N. G. Terry, E. S. Dellon, and N. J. Shaheen, Gastroenterol., 141, 443–447 (2011).
PERRY A. GENOVA, Ph.D., is president and CEO of Oncoscope Inc., Durham, NC; e-mail: [email protected]; www.oncoscope.com.
The a/LCI system setup
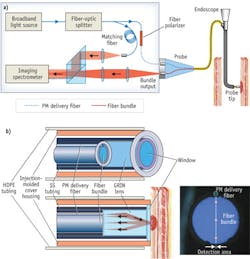
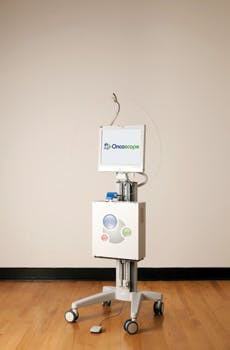
Oncoscope's a/LCI system consists of an optical engine with an attached fiber-optic probe and a single-use sterile disposable cover to facilitate rapid turnaround in high-volume endoscopy centers (see Fig. 1). A computer running proprietary software controls the optical engine, analyzes data, and communicates with the user via a custom graphical user interface (GUI), touch-screen keyboard, and foot switch. The optical engine, computer, and foot switch are housed on a portable cart for easy maneuverability within and between endoscopy suites.
The fiber-optic probe can be connected and disconnected from the optical engine with a single-handed motion. The probe consists of an individual optical fiber that delivers near-infrared light to the distal optics, where it is collimated for illumination of the esophageal tissue (see Fig. 3b). The optics setup collects light scattered from nuclei and other tissue structures, and separates that light as a function of scattering angle into an array of optical fibers for delivery back to the optical engine for detection and analysis.
The core of the system is based on a modified Mach-Zehnder interferometer (see Fig. 3a, in article). Light from a superluminescent diode (SLD; 830 nm ± 20 nm) is split into sample and reference arms by a fiber splitter. The sample-arm light, delivered to the tissue and collected by the system probe, is then relayed by a 4f optical train to the input slit of an imaging spectrograph. Just before the scattered light enters the spectrometer, a holographic beamsplitter recombines it with light from the reference arm—which is matched by path length to the fundamental mode of the sample arm. A high-resolution grating disperses the mixed fields by wavelength, and an integrated, cooled CCD camera detects them. This setup effectively performs Fourier-domain interferometry, but with the captured light resolved by scattering angle.
The detected image uploads to the system computer for processing and analysis. Consistent with Fourier-domain interferometry, the Fourier transform of the interferogram for each scattering angle converts the wavelength dimension to depth within the tissue. This effectively makes the interferogram an OCT A-line specific to each of the scattering angles.
The system compiles angular scattering distributions from depths corresponding to various regions within the Barrett's epithelium across the A-lines, and then analyzes them to detect signatures in the angular scattering patterns indicative of dysplasia. The GUI immediately displays analysis results along with recommendations as to whether or not an excisional biopsy should be acquired at the measurement site and submitted for histological processing and evaluation (see Fig. 4, in article).