Hybrid gold nanoparticles could pair cancer imaging, therapy
Researchers at Imperial College London (England) and the French National Center for Scientific Research (CNRS; Paris, France) have designed and developed hybrid gold-silica nanoparticles that make it possible to combine two forms of tumor treatment with three bioimaging techniques. Tested in mice and on cultured human cells, the nanoparticles demonstrated greater drug loading and delivery capacity than other carriers available, which is promising for cancer research.
Related: Highly specific fluorescent nanoparticles could enable personalized medicine
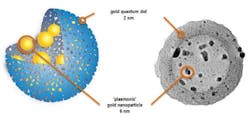
The tool developed by the research team combines magnetic resonance imaging (MRI), near-infrared (NIR) fluorescence, and photoacoustic imaging with two forms of therapy (chemotherapy and photothermal therapy), all within a sphere measuring 150 nm in diameter. To achieve this, the researchers synthesized hybrid objects consisting of a mesoporous silica shell containing gold quantum dots—small nanoparticles (less than 2 nm) with unique properties (fluorescence, heat production, and magnetism) that are very different from those of solid gold, or even larger gold nanoparticles. However, their lack of stability in aqueous solvents (they tend to aggregate to form larger particles) had prevented their use in biology and medicine until now. By "infusing" porous silica shells with gold precursors, researchers succeeded in creating gold quantum dots in the pores of the shell (which stabilizes them), as well as larger gold nanoparticles in the central cavity. Stable in aqueous solutions, this "quantum rattle" structure can penetrate into the center of cells without toxicity. It also preserves the optical and magnetic properties of gold quantum dots, while maximizing their drug storage capacity.
The incorporation of hydrophobic gold in the silica sphere helped to very significantly increase its storage capacity for doxorubicin, an anticancer agent often difficult to stabilize in this type of porous matrix. The scientists believe that the proportion of molecules that would reach their target would rocket from 5 to 95 percent, in comparison to (liposomal-type) drug carriers currently available. In addition to this capacity to carry drugs, they have potential in photothermic therapy. In fact, when they are excited by an IR laser, the particles containing the gold quantum dots emit IR fluorescence, but also enough heat—up to 51°C—to kill cancerous cells. This made it possible to reduce tumor mass in mice by 55 percent after a single treatment.
The production of heat can also be used for imaging purposes, as it causes a temporary dilation of gold quantum dots—this produces ultrasound waves that can be detected, as in ultrasound imaging. Moreover, the fluorescence emitted by the laser-excited particles travels through tissue (which does not absorb infrared in this wavelength), and can therefore be measured in a noninvasive manner. Finally, for sizes smaller than 2 nm, gold becomes magnetic. It is therefore possible to use quantum rattles as a contrast agent for MRI. These three imaging methods (NIR fluorescence, photoacoustic imaging, and MRI) make it possible to observe the tumor in complementary ways, with very high spatial and temporal resolution.
The researchers would like to "functionalize" their surface with markers so that they can identify and specifically target cancerous cells. Finally, they hope to be able to reduce the size of the gold particles in the central cavity to make the carrier entirely biodegradable.
Full details of the work appear in the Proceedings of the National Academy of Sciences (PNAS); for more information, please visit http://dx.doi.org/10.1073/pnas.1419622112.
-----
Follow us on Twitter, 'like' us on Facebook, connect with us on Google+, and join our group on LinkedIn