Optical Coherence Tomography/Fluorescence Imaging: Fiber assembly enables efficient multimodal endoscopy
KATHY BEAUDETTE
While optical coherence tomography (OCT) provides structural contrast with a resolution on the order of tens of microns, for some applications this information is, in some contexts, insufficient to provide a definitive diagnostic. To mitigate this limitation, OCT has been used in conjunction with fluorescence imaging or spectroscopy for detection of molecular content in tissues. The combination of OCT and fluorescence has been used for a variety of applications, most notably to study atherosclerotic plaque in cardiology,1 to visualize the fine vascular network in pulmonology (for which autofluorescence is used),2 and for cancer imaging.3
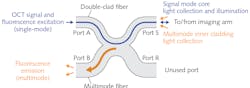
For endoscopic applications, a single-fiber scheme is typically preferred. Double-clad fiber (DCF) enables integration of OCT and fluorescence modalities, offering a single-mode core that allows propagation of a fundamental mode, surrounded by an inner cladding that supports the propagation of multimode light, and then an outer cladding. The core can be used to propagate the OCT signal to the sample and back to the detector. Because the inner cladding has a large numerical aperture (NA), it can efficiently collect the diffuse photons from fluorescence or spectroscopy. Though fluorescence excitation can be sent to the sample through either the core or the inner cladding, using the core for this purpose has been shown to provide a better fluorescence resolution.2
Couplers are key
To decouple the OCT signal from the fluorescence emission coming back from the sample, a fiber-based configuration is preferred, as it provides a robust, alignment-free system—which is especially critical for clinical devices. Double-clad fiber couplers (DCFCs) were developed to fulfill this purpose.4,5 DCFCs allow for quasi-lossless transmission of a single-mode signal (insertion loss <0.5 dB), typically the OCT signal, through the core of the DCF. By fusing and tapering the DCF with a specially selected multimode fiber (MMF), it is possible to achieve an efficient (up to 75%) multimode transfer of the collected fluorescence emission from the inner cladding of the DCF to the core of the MMF (see Fig. 1).
Typically, Port A of the DCFC is connected to a wavelength division multiplexer (WDM), which combines the OCT signal and fluorescence excitation light, and Port S is connected to the imaging arm, which includes a DCF-based catheter. Port B collects fluorescence emission and is connected to a detector such as a photomultiplier tube (PMT). Previously unavailable commercially, DCFCs are now manufactured by Castor Optics for a wide range of single-mode wavelength bands and available through Thorlabs.
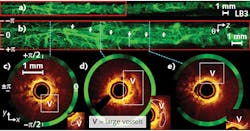
Results obtained using a DCFC-based system that combines OCT and autofluorescence for endoscopic imaging of airways are shown in Figure 2. The fiber implementation allows high-resolution and high-sensitivity autofluorescence detection concurrent with high-sensitivity OCT imaging.Seamless integration
To assist the work of researchers and engineers seeking to integrate fluorescence detection within existing OCT systems, a line of “plug-and-play” modules based on Castor Optics’ core DCFC technology and tailored for high-sensitivity biomedical imaging is now available for a variety of OCT central wavelengths: 780, 1060, and 1300 nm. Every component is carefully selected and high-quality splicing is performed to minimize insertion loss for both the fluorescence and OCT paths. This last aspect is especially critical in such systems since different fiber types (DCFs, and single- and multimode fibers) are used. The fiber network is neatly packaged in a compact unit.
A sample configuration is a dual-balanced interferometer compatible with a 1300 nm swept-source OCT system that includes a WDM to combine OCT signal with the excitation laser within the DCF core (see Fig. 3a). The WDM transmits the OCT signal with minimal loss while acting as a short-pass filter for the visible excitation laser. Figure 3b shows a typical curve of the insertion loss as a function of wavelength for the fluorescence excitation path. The same unit can be used for a wide wavelength range of excitation lasers, from 405 to 980 nm. The OCT path contains a first splitter, to split the signal between the reference and sample arms, circulators, and a 50:50 splitter connected externally to a dual-balanced detector. In the sample arm, a DCFC is used to deliver the OCT signal to the imaging arm through the core. Fluorescence emission from the sample is collected primarily by the large NA inner cladding such that, on the trip back, it will be collected by the multimode fiber port of the DCFC. The multimode transfer response of the DCFC is fairly flat for a large wavelength range (see Fig. 3c).Thus far, DCFCs have been applied in biomedical imaging primarily to combine OCT and fluorescence modalities. However, the technology can also be used for various other modalities such as confocal endoscopy, nonlinear imaging, sensing, and concurrent OCT and laser tissue coagulation. DCFCs allow for a complete fiber-based implementation, enabling a compact and alignment-free system ideal for endoscopic and clinical applications. More and more turnkey solutions are available, allowing the user to integrate the technology within an existing imaging system without hassle.
REFERENCES
1. H. Yoo et al., Nat. Med., 17, 12, 1680–1684 (2011).
2. H. Pahlevaninezhad et al., Opt. Lett., 41, 14, 3209–3212 (2016).
3. F. Feroldi et al., Biomed. Opt. Express, 9, 12, 6186–6204 (2018).
4. S. Lemire-Renaud et al., Opt. Express, 18, 10, 9755–9764 (2010).
5. W.-J. Madore et al., Opt. Lett., 38, 21, 4514–4517 (2013).
Kathy Beaudette is product line manager at Castor Optics, Montréal, QC, Canada; e-mail: [email protected]; www.castoroptics.com.
![FIGURE 3. A typical interferometer module schematic for swept-source OCT with dual-balanced detection is shown (a), including a packaged interferometer module example (inset), a typical insertion loss plot of a short-pass WDM ([b]; excitation laser to DCFC), and a typical multimode transfer as a function of wavelength of a DCFC ([c]; sample to emission detection). FIGURE 3. A typical interferometer module schematic for swept-source OCT with dual-balanced detection is shown (a), including a packaged interferometer module example (inset), a typical insertion loss plot of a short-pass WDM ([b]; excitation laser to DCFC), and a typical multimode transfer as a function of wavelength of a DCFC ([c]; sample to emission detection).](https://img.laserfocusworld.com/files/base/ebm/lfw/image/2019/08/1908LFW_bea_f3.5d49cb8537130.png?auto=format,compress&fit=max&q=45?w=250&width=250)