Deep-tissue Imaging: Small-molecule NIR-II pushes in vivo imaging deeper
While living tissue strongly absorbs and scatters visible light, there are two spectral regions at slightly longer wavelengths for which attenuation and scattering is lower. These two bands are called the first and second near-infrared (NIR) windows. The NIR-II band is particularly attractive, although labels that emit in this band are difficult to fabricate. A team of researchers at Stanford University (Palo Alto, CA) and Wuhan University (Hubei, China), however, has developed a biocompatible organic small-molecule NIR-II dye with improved quantum yield.1
The NIR-I window covers from about 700 to 900 nm, while the NIR-II window is open from about 1000 to 1800 nm. Because scattering generally diminishes with longer wavelengths, autofluorescence (fluorescence signals from native molecules) is much lower in NIR-II. This gives NIR-II’s signal-to-noise ratio the potential to be high, even while its low attenuation facilitates deep imaging. Just because there’s a window, though, does not mean that there are tools (in this case, appropriate fluorophores) to open it. It’s not for lack of trying, though, and recent reviews have summarized efforts to develop dyes based on lanthanide nanoparticles, conjugated polymers, small-molecules, carbon nanotubes, and other mechanisms. The challenge is rooted in fundamental physics.
Excitation-state overlap
Visible light interacts with matter primarily through changes in electronic energy levels. That is, the energy contained in a visible photon matches the difference in energy between a ground state and excited state of an electron in a molecule in the material. Infrared wavelengths—say, 5 µm and longer—interact with vibrational states of a molecule. That is, the much-lower energy in an IR photon matches, for example, the difference between a low-energy stretch in a carbon-hydrogen bond and a more-energetic vibration in that bond.
Visible light changes the motion of an electron “internal” to a molecule, while IR light changes the motion of an atom accessible to the surrounding environment. Vibrational or rotational energy corresponds to temperature. Infrared absorption, then, raises the temperature of matter.
The NIR-II window lies right between these extremes, and this is where the problem arises. A NIR photon is absorbed and raises the energy of an electronic state. But, because NIR photons have lower energy than visible photons, the excited electronic state overlaps with vibrational states of the absorbing molecule. A photon comes in, but that energy doesn’t need to come out in the form of another photon. The overlap of the vibrational states, in fact, means that the photon energy is much more likely to lead to heating, and nonradiative transfer to the environment.
The quantum yield is defined as the fraction of photons out to photons in. Nonradiative decay is more likely than radiative decay, so the quantum yield for NIR-II dyes is generally very low.
Mikhail Berezin, a professor of radiology at Washington University in Saint Louis, works developing NIR and SWIR bioimaging techniques. “We’d like to have as high a quantum yield as possible,” he says. “Physics tells us that the longer the wavelength, the easier to heat, so we don’t want to give the labeling molecules any freedom to vibrate.”
Stiffening a small molecule fluorophore
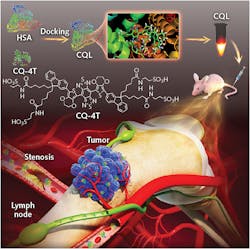
The Stanford-Wuhan team pursued a small-molecule approach (see figure). Small molecules are by design nontoxic and easily cleared from biological tissues. The team modified a previous NIR-II fluorophore design they had developed, increasing the quantum yield and simplifying the chemical synthesis. Still, the quantum yield was only about 0.1%.
The next trick was to dock the fluorescent molecule to human serum albumin (HSA). HSA has excellent in vivo stability and is a normal component of blood, so it’s eminently biocompatible. Fitting the small fluorescent molecule into the HSA worked to “stiffen” the molecule. That essentially raises the energy of some of the vibrational modes, putting them out of the reach of the energy of the excitation photon. That reduces some of the heating, and raises the quantum yield to more than 0.3%.
To determine if this quantum yield was sufficient for deep imaging, the team injected nude mice with the dye complex and imaged the blood flow. They found the brightness and quantum yield sufficient to allow fast wide-field acquisition, including imaging high-order branches of blood vessels. After the successful proof-of-principle imaging, the team simulated some clinical applications. Their label revealed revascularization associated with a tumor, for example, and, when injected into the lymphatic system, was able to guide precise resection of both a sentinel lymph node and a secondary lymph node.
The initial results are promising, but Daifeng Li, lead researcher on the study, cautions that “the development of small-molecule NIR-II probes is still in its infancy.”
REFERENCE
1. D.F. Li et al., Adv. Funct. Mater., 2019, 1906343 (2019); https://doi.org/10.1002/adfm.201906343.

Richard Gaughan | Contributing Writer, BioOptics World
Richard Gaughan is the Owner of Mountain Optical Systems and a contributing writer for BioOptics World.