NICK RUSZKOWSKI and GIULIA OSSATO
Advances in optical microscopy are enabling biological imaging in important ways, from the lab to the operating theater.
Better brain surgery
Beyond improving the view through magnification, resolution, and illumination, microscopy that enables live, intraoperative imaging helps optimize the chances of success in critical procedures such as neurosurgery.
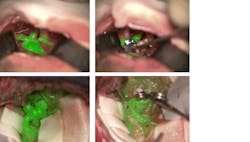
The ability to visualize blood vessels and blood flow is important to surgeons, and one of the latest innovations in surgical imaging is a device that allows the anatomic microscope to produce excitation light and resolve fluorescence emission from indocyanine green (ICG), an agent that fluoresces under near-infrared (near-IR) light. The device is a noncontact, noninvasive augmented-reality (AR) microscope accessory that digitally combines two video streams, one from a camera that captures visible light images and another from a camera that captures near-IR fluorescence angiography information. The result is a high-definition image of cerebral anatomy in natural color, augmented by an overlay of real-time pseudo-colored vascular blood flow.
In the traditional application of ICG for blood vessel and blood flow visualization, the surgeon must pause the operation, watch a separate black-and-white video, and then recall and reconcile this information with the anatomic microscope view. The new innovation, offered in the Leica GLOW800 surgical microscope accessory, provides both angiographic and anatomical information in the same field of view, with full depth perception (see figure).
Another innovation targeting surgical interventions is a microscope filter—designed to provide intense, homogenous excitation light and a well-adjusted observation spectrum—that works with the active substance 5 aminolevulinic acid (ALA) to enable visual differentiation of malignant glioma tissue from healthy brain tissue in real time. Therefore, it supports decision-making in some of the most complex and critical neurosurgical interventions, and aids precise tumor resection, which is vital to preserve brain function and extend life. Combined with a digital AR microscope—one such as Leica’s ARveo that provides a precise, integrated view of the surgical field in a single platform—the filter (Leica FL400) gives surgeons real-time, bright, and high-contrast delineation of tumor margins. A button touch reveals the fluoresced tissue, supporting the smooth running of an intrasurgical workflow and giving surgeons the option to operate “heads-up.”
Functional imaging
A technique that holds great potential for the imaging of cellular metabolism or microenvironmental changes is fluorescence lifetime imaging (FLIM), which produces an image based on the time that fluorophores spend in the excited state. In this approach, the duration of the fluorophore signal, rather than its intensity, is used to determine the color of each pixel. Because fluorescence lifetime is independent of fluorophore concentration, it can be used for making chemical measurements, such as of the pH of the local molecular environment. Given these characteristics, FLIM allows biomolecular functional imaging.
Further, FLIM can be used to observe and measure Förster resonance energy transfer (FRET), an approach popular for visualizing molecular interactions and for biosensing. In combination with two-photon excitation, FLIM can be exploited to study metabolic processes without the need for labeling by analyzing intrinsic fluorescence—of nicotinamide adenine dinucleotide (NADH), vitamins and other key biological cofactors.
Despite these powerful capabilities, the application of FLIM has been limited. Several factors account for this, such as the intrinsically slow and difficult process required to implement the traditional time-correlated single photon counting (TCSPC) solutions, particularly for complex imaging workflows. As a result, FLIM has mainly been relegated to specialized laboratories and, even with expert knowledge, traditional TCSPC has been unable to deliver the speed needed to observe biological processes occurring at time scales below tens of seconds.
Commercial developers are working to change this, however. To help scientists investigate dynamic physiology in living cells, for instance, a high-speed, fully integrated confocal system (SP8 FALCON for FLIM) not only broadens access to complex FLIM experiments, but also enables new kinds of research, including biosensing of changes in cellular metabolic states and microenvironments, and the tracking of fast molecular interactions between proteins (such as receptor signaling).
Digital refinements can also greatly improve the resolving power of microscopy. For example, the LIGHTNING Image Information Extraction method is a fully automated approach that incorporates adaptive deconvolution to enable greater resolution than can usually be achieved using classical deconvolution methods. Deconvolution is employed to remove out-of-focus signal—not by discarding them, but by reassigning signals to their original positions, thus preserving the total signal or photon counts in the imaging volume. By using intelligent adaptive procedures for image enhancement, based on a priori knowledge from the imaging system, it is possible to achieve resolution of 120 nm—and even finer resolution can be reached using stimulated emission depletion (STED) nanoscopy. Research into this approach has so far achieved resolutions as low as 30 nm.1
Microscopy is already being used to detect cancer cells among healthy ones—for example, with multiphoton microscopy in the diagnosis of malignant melanoma.2-4 Life scientists are also using FLIM-FRET to measure the spatiotemporal dynamics of signaling activity in live neurons,5 where light scattering by brain tissue can be problematic for other imaging methods. These are just two examples of specific applications where FLIM and FLIM-FRET offer advantages and possibilities over conventional confocal imaging. It is anticipated that FLIM will become a standard tool for investigating biological processes and cellular microenvironments, enabling novel treatments for disease. And of course, continued advances in optics and photonics will enable further system developments.
REFERENCES
1. J. Hanne et al., Nat. Commun., 6, 7127 (2015).
2. E. Yew, C. Rowlands, and P. T. C. So, J. Innov. Opt. Health Sci., 7, 1330010 (2014).
3. G. Lentsch et al., Pigment Cell Melanoma Res., 32, 403–411 (2019).
4. K. König (Ed.), Multiphoton microscopy and fluorescence lifetime imaging – applications in biology and medicine, De Gruyter, ISBN: 978-3-11-042998-5 (2018).
5. R. Yasuda, Curr. Opin. Neurobiol., 16, 551–561 (2006).
Nick Ruszkowski is Global Commercial Strategy Manager and Giulia Ossato, Ph.D., is Product Manager Confocal, both at Leica Microsystems, Buffalo Grove, IL and Mannheim, Germany; e-mails: [email protected] and [email protected]; www.leica-microsystems.com.