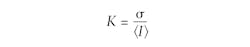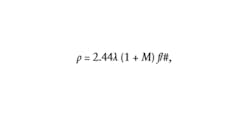Stabilized diodes enable improved laser speckle contrast imaging
Laser speckle is an interference phenomenon resulting from optical path-length differences associated with surface roughness. Speckle in diffusely reflected laser beams produce a seemingly random distribution of bright spots (constructive interference) and dark spots (destructive interference) within the laser-illuminated area. Lasers are rarely used as illumination sources in conventional imaging applications because speckle adds unwanted noise in the image. That said, there are some situations where valuable information can be obtained by carefully analyzing the speckle pattern in the image—for example, surface roughness and dynamic flow.
Laser speckle contrast imaging (LSCI) is an imaging tool in which speckle patterns in dynamic systems are analyzed to detect flow. LSCI takes advantage of the fact that during long exposure time imaging of dynamic systems, the particle movement results in temporal variations that blur the speckle pattern, resulting in decreased contrast (also referred to as fringe visibility). In isolation, this doesn’t offer very much information, but for objects containing a spatial distribution of flows, the difference in contrast directly correlates to the relative flow rate. LSCI is particularly useful when measuring blood flow in living tissue during both surgical and noninvasive procedures, as it is common to have areas of high flow adjacent to static regions.
LSCI has been actively studied for over 20 years but, until recently, surprisingly little research had been done to understand the effects of laser parameters on image quality. One possible reason for the lack of interest in this topic was the assumption that since the interaction path was relatively small, any laser with a minimum coherence length of roughly 100 μm would suffice. New studies have shown that the laser characteristics have far more impact on the quality of LSCI images than previously thought, especially wavelength and linewidth. This article will review these recent developments to serve as guidance when selecting an illumination laser for LSCI, in addition to highlighting some LSCI applications that are benefiting as a result.
How is speckle contrast calculated?
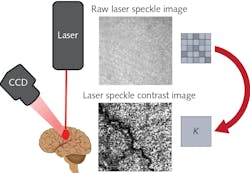
To understand these relevant laser parameters, it is essential first to understand the basic image-processing methodology behind LSCI. Figure 1 shows a schematic representation of a typical LSCI setup in which a laser illuminates the region of interest and a monochromatic camera images the diffusely reflected speckle pattern. Next, the image pixels are binned into small subgroups (e.g., 5 × 5 pixel arrays) over which the speckle contrast (K) is calculated using the equation:In this equation, σ represents the pixel intensity standard deviation and 〈I〉 represents the average pixel intensity within the subgroup. The greater the speckle contrast within the subgroup, the larger the value of K. The converse is true as well: the lower contrast, the smaller the value of K. Therefore, K provides a quantitative measurement of the speckle contrast and allows for a new image to be produced in which each pixel represents the contrast of a particular subgroup. For a more detailed analysis of how speckle contrast correlates to flow rate, Boas and Dunn’s 2010 review article titled “Laser Speckle Contrast Imaging in Biomedical Optics,” published in the Journal of Biomedical Optics, is a valuable resource.1
How to choose the right laser
With this understanding of how to produce speckle contrast images, it’s easy to see that the most important considerations are speckle size, illumination uniformity, and depth penetration. If not uniformly illuminated, the area of interest will result in variations in the average intensity across the image plane. Additionally, if the average speckle size is too large, it will require a larger binning window to detect the variance, resulting in further image degradation and loss of resolution. Lastly, if the depth penetration is too shallow, the results will not adequately represent the blood flow below the surface of the tissue.
Illumination uniformity has mostly been solved using large-core multimode fiber optics and stationary ground-glass diffusers to produce relatively flat-top beam profiles. These two easily implementable approaches virtually eliminate the issues related to “hot spots” and “dark spots” associated with traditional free-space laser beam profiles (both single- and multimode), while still maintaining sufficient spatial coherence. Additionally, the use of multimode fiber coupling allows for added flexibility when designing LSCI instrumentation. Fiber delivery is incredibly helpful for developing commercial LSCI instrumentation for intraoperative use.
The other two considerations, speckle size and depth penetration, are more complicated to address since they tend to have conflicting requirements. For example, Boas and Dunn showed that the minimum speckle size is directly proportional to the laser wavelength,1 according to the following equation:where M represents the system magnification. One could assume the shortest possible wavelength for the illumination laser is best; however, depth penetration is inversely proportional to wavelength. Therefore, if the wavelength is too short, the photons will be absorbed at or near the surface and won’t ever penetrate deep enough to scatter off the blood cells
While working on his master’s thesis at Miami University (Oxford, OH), Anthony Young performed a fairly comprehensive study of this issue, looking at the effects of laser wavelength and polarization on depth sensitivity in LSCI.2-4 In his research, Young worked with four different lasers: a 785 nm diode laser from Innovative Photonic Solutions (Monmouth Junction, NJ), a 633 nm HeNe laser from Melles Griot (Carlsbad, CA), and a 532 nm diode-pumped solid-state (DPSS) laser and 405 nm diode laser both from AixiZ (Houston, TX).
In his initial work on post-occlusive reactive hyperemia, Young observed that, while the 405 nm laser provided the highest spatial resolution, the limited depth penetration led to erroneous flow readings. He then examined the depth sensitivity by using a phantom to study LSCI at various wavelengths under controlled conditions. He found that, despite the reduction of spatial resolution, 785 nm tended to provide the highest sensitivity to flow at all tested depths. This result is most likely due to the minima commonly observed in the near-infrared region of the absorbance spectrum of mammalian tissue.
Additional work performed by Dmitry Postnov et al. at Boston University (Boston, MA) showed that, in addition to the peak wavelength of the laser source, wavelength stability is also extremely important.5 Unexpectantly, the research showed a 50% increase in global speckle contrast between stabilized and unstabilized 785 nm diode lasers—Kg = 0.83 and Kg = 0.59, respectively.
As discussed previously, it was believed that any coherence length longer than 100 μm (which correlates to a linewidth of less than 2 nm at 785 nm) would produce a contrast greater than K = 0.8. Based on their experiments, Postnov et al. concluded that laser sources for LSCI should instead have a linewidth of less than 0.5 nm, which correlates to a coherence length of roughly 400 μm.
They additionally recommend the use of a volume holographic grating (VHG), also known as a volume Bragg grating (VBG), as an efficient means of laser-diode stabilization. Figure 2 shows an example of a multimode gallium arsenide (GaAs) laser diode before and after VBG stabilization, measured using an optical spectrum analyzer from Ando Electric Co. (Tokyo, Japan). This data, provided by Innovative Photonic Solutions, indicates that VBG stabilization reduced the linewidth from 0.971 nm to 0.151 nm, making it more suitable as an LSCI illumination source.The current state of LSCI technology
Today, there are several commercially available LSCI systems available that take advantage of the benefits of 785 nm laser diodes, such as the MoorFLPI from Moor Instruments Ltd. (Axminster, England) and Pericam PSI from Perimed AB (Järfälla, Sweden). These systems are currently used in clinical applications such as blood-flow monitoring in burn assessment,6 laparoscopic colorectal resection,7 and Ivor-Lewis esophagectomy.8 There is also a great deal of research into new LSCI modalities. Some recent developments include the integration of LCSI directly into lipotropic probes9 and dual-wavelength LSCI systems designed to measure flow and blood oxygenation simultaneously.10 As LSCI technology continues to advance, and more applications transition from the research environment, stabilized near-infrared lasers will continue to be at the forefront of LSCI technology.
REFERENCES
1. D. A. Boas and A. K. Dunn, J. Biomed. Opt., 15, 1, 011109 (2010).
2. A. M. Young, “Investigation of laser speckle contrast imaging’s sensitivity to flow,” Master’s Thesis, Miami University (2018).
3. A. Young and K. Vishwanath, Proc. SPIE, 10573, 105732Z (Mar. 2018).
4. A. Young and K. Vishwanath, Proc. SPIE, 9707, 97071H (Mar. 2016).
5. D. D. Postnov et al., Sci. Rep., 9, 1, 1–6 (2019).
6. R. Mirdell et al., Burns, 45, 6, 1325–1335 (2019).
7. S. Kojima et al., Asian J. Endosc. Surg., 13, 3, 329–335 (2020).
8. R. Ambrus et al., Scand. J. Gastroenterol., 52, 4, 455–461 (2016).
9. C. Zheng et al., Biomed. Opt. Express, 9, 12, 5962–5981 (2018).
10. C. Y. Lee et al., OSA Continuum, 3, 5, 1129–1137 (2020).
About the Author
Robert V. Chimenti
Director, RVC Photonics LLC
Robert V. Chimenti is the Director of RVC Photonics LLC (Pitman, NJ), as well as a Visiting Assistant Professor in the Department of Physics and Astronomy at Rowan University (Glassboro, NJ). He has earned undergraduate degrees in physics, photonics, and business administration, as well as an M.S. in Electro-Optics from the University of Dayton. Over a nearly 20-year career in optics and photonics, he has primarily focused on the development of new laser and spectroscopy applications, with a heavy emphasis on vibrational spectroscopy. He is also very heavily involved in the Federation of Analytical Chemistry and Spectroscopy Societies (FACSS), where he has served for several years as the Workshops Chair for the annual SciX conference and will be taking over as General Chair for the 2021 SciX conference.