New targets for OCT in inspection and metrology
Optical coherence tomography (OCT) is a 3D biomedical imaging technique with micron-scale resolution and millimeter penetration depth. Its greatest application is in ophthalmology, where it is used to reveal the layered structure of the retina. It is also used to image other biological tissues such as skin, epithelium, and vasculature. But as the technique has advanced and instrumentation becomes more widely available, a range of new targets for OCT are emerging.
From the start, the developers of OCT hypothesized that the optical technique could aid in manufacturing processes for glass/polymer composites.1 The technique seemed like an opportunity to avoid wasting product for quality inspection. Nondestructive inspection was in its infancy and OCT hoped to join the ranks of production-line tools.
But compared to ophthalmology, commercialization of the technology in this area was slow to nonexistent. Traditional OCT machines are bulky and expensive. OCT inspection is limited by the optical properties of substances under inspection. Dark paint or opaque glue blocks any light from returning to the sample, and metal is out of the question. These limitations do not make OCT the first choice for nondestructive inspection for many manufacturers, but demand for better resolution has motivated innovative visualization techniques.
From inspecting lenses in vivo to offline inspection
A recent application for OCT in-line inspection is familiar territory in ophthalmology: contact lenses. Contact lenses are an ideal candidate for OCT inspection because their film is transparent. Several studies assess contact lenses in vivo for fit and comfort. Contact lenses are made of oxygen-permeable plastics and, similar to the human eye, relevant features are on the micron scale—contact lenses are approximately 400 µm thick.
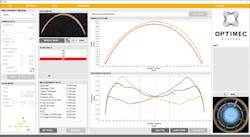
Measuring contact lenses can be challenging due to their geometry, which require an extended depth OCT system with approximately 2 mm of imaging range to visualize the entire surface. Geometric inspection is the current process for evaluating contact lens quality. But a 2016 study demonstrated that OCT inspection tools, including the OPTIMEC is830 (see Fig. 1) by Optimec Systems (Gloucestershire, U.K.), improve the accuracy of key measurements.2 Although this is a system designed to aid in verifying manufactured lenses during development, higher throughput is needed to enable product inspection.
Analyzing coating deposition
OCT can enable real-time visualization of pill coatings with micron-scale thickness. Preliminary studies have shown the ability to measure coatings with high precision, but there are limitations: opaque pill coatings can prevent thickness measurements and the curvature of a pill may prevent complete imaging without rotating the pill or using multiple orientations for imaging. Recent efforts in this area focused on how to integrate OCT into the manufacturing chain.
A 2020 report presents an OCT imaging system adapted to rapidly visualize pills within a rotating drum.3 The drum contained perforations that enabled OCT measurements of a pill within the perforations. By rapidly rotating the drum, the pills are moved past the imaging area and coatings can be characterized in line. Parameters such as coating thickness, homogeneity, and roughness are obtained through automated image analysis.
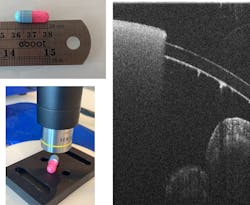
A commercial version of this system, the OseeT from Phyllon (see Fig. 2), was developed. Some pill coatings are transparent, while others remain opaque. Figure 3 shows a pill with different optical properties on each side.Although a single offline measurement is feasible to verify batch quality, OCT shows potential to be developed as an inline monitoring technology as well, as long as the images are obtained and analyzed quickly enough to be used as feedback on the process. Other examples of OCT monitoring deposition include examination of indium tin oxide (ITO) coatings4 and monitoring of laser-based metal deposition.5
OCT for dental repair
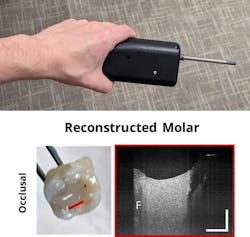
OCT has been explored in dentistry, because the 3D imaging technique can visualize morphological changes in the surface of hard tissues. But it can also be used to assess the features of fabricated dental structures, such as dentures.6 To evaluate the feasibility of examining dental structures both in vivo and during fabrication, Lumedica developed a handheld OCT probe with support from the U.S. National Science Foundation. Figure 5 shows the probe alongside a reconstructed molar. The composite filling is clearly visible and the contact with the remaining tooth can be well defined and evaluated.3D rendering changes the picture
In 2023, Lumedica will launch the OQ LabScope 3.0. The system retains the same hallmarks of Lumedica design, including an affordable OCT system, and it features a faster A-scan rate and capability for 3D rendering to standard systems. Its improved speed makes 3D rendering within the software more practical—a full volume scan of a 5 × 5 × 7 mm3 area takes mere seconds.
The OQ LabScope 3.0 contains all components for an OCT system, including a spectrometer, computer, and imaging software within a single platform. Its light source is an 840-nm SLD, which achieves an axial resolution of 6 µm in tissue. Flexible software allows users to set the size for all scan types, including horizontal, vertical, radial, circle, and volume scans (up to 5 × 5 mm2).
With these powerful imaging systems, new applications in inspection and metrology can be explored and realized. Continual innovation in OCT system design, while also reducing cost and increasing utility and flexibility, sets the stage for the next phase of OCT applications.
REFERENCES
1. J. P. Dunkers et al., Compos. - A: Appl. Sci. Manuf., 30, 2, 139–145 (1999).
2. B. J. Coldrick, C. Richards, K. Sugden, J. S. Wolffsohn, and T. E. Drew, Cont. Lens Anterior Eye, 39, 4, 270–276 (Aug. 2016); doi:10.1016/j.clae.2016.01.002.
3. S. Sacher, A. Peter, and J. G. Khinast, Int. J. Pharm. X, 3, 100067 (Dec. 17, 2020); doi:10.1016/j.ijpx.2020.100067.
4. M.-T. Tsai et al., Opt. Express, 19, 7559–7566 (2011); https://opg.optica.org/oe/fulltext.cfm?uri=oe-19-8-7559&id=211606.
5. C. Stehmar, M. Gipperich, M. Kogel-Hollacher, A. Velazquez Iturbide, and R. H. Schmitt, Appl. Sci., 12, 5, 2701 (2022); https://doi.org/10.3390/app12052701.
6. Y. Sumi, N. Ozawa, S. Nagaosa, S. Minakuchi, and O. Umemura, Arch. Gerontol. Geriatr., 53, 2, 237–241 (2011).
About the Author
Adam P. Wax
Co-founder and President, Lumedica
Adam P. Wax, Ph.D., is co-founder and president of Lumedica; a Professor of Biomedical Engineering at Duke University, a faculty network member of the Duke Institute for Brain Sciences; and a member of the Duke Cancer Institute. His research interests include optical spectroscopy for early cancer detection, novel microscopy, and interferometry techniques. Dr. Wax is a Fellow with the American Institute for Medical and Biological Engineering, the International Society for Optics and Photonics, and the Optical Society of America.
He holds 5 patents, and his technology innovations led to his founding of Oncoscope, Inc. where serves as Chairman. Dr. Wax received a B.S. in electrical engineering from Rensselaer Polytechnic Institute, a B.S. in physics from SUNY Albany, an MA and a Ph.D. in physics from Duke University, and completed his postdoctoral training at MIT.
McKenzie Peterson
Sales and Marketing Associate, Lumedica
McKenzie Peterson is a sales and marketing associate at Lumedica (Durham, NC).