Image-guided Surgery: Optical Surgical Navigation group targets meaningful tool comparison
With a goal of helping surgeons target cancerous tumors with greater precision, optical imaging has begun to enable objective, intraoperative assessment of tissues, cells, and biochemical events.
Traditionally, surgeons have had only subjective means (palpation, visual inspection, and experience) for determining the location of tumors. But because such tools are unable to delineate tumor margins, they produce high rates of both positive margins and cancer recurrence in patients. By contrast, optical image-guided surgery has been shown to reduce the positive margin rate and improve patient outcomes.
At this point, multiple optical modalities—including both label-free and fluorescence approaches—have achieved clinical translation. In fact, recent years have seen an explosion in clinical trials for optical image-guided surgery, as more than 150 are listed on ClinicalTrials.gov. These setups involve such building blocks as light sources, CCD cameras, fluorescent probes, and ultraminiaturized microscopes.
Given their wide variety, the range of parameters they detect, and the fact that progress requires interdisciplinary coordination (of biology, chemistry, engineering, and medicine), the World Molecular Imaging Society (WMIS) has identified a need for review, discussion, refinement, and prioritization of methods. Their answer to this need is the establishment of an Optical Surgical Navigation (OSN) interest group, which aims to develop instrumentation standards and pursue full disclosure of parameters and specifications. The OSN reports that these steps will enable performance comparison of novel probes and imaging systems.
New clinical emphasis
The OSN is strengthened by the WMIS's 2016 acquisition of the American Society of Image Guided Surgery (ASIGS), which was established to bring together oncologic surgeons to discuss advances in this area. ASIGS continues to operate—and to maintain its identity—within OSN. The pairing is complementary: ASIGS has traditionally included mainly surgeons interested in the clinical translation of novel agents, while OSN, with a focus on basic science and preclinical applications, has attended primarily to the development of molecular imaging agents and hardware.
OSN was started by Michael F. Tweedle, Ph.D. (The Ohio State University; Columbus, OH), and James P. Basilion, Ph.D. (Case Western Reserve University; Cleveland, OH), who serve as the group's co-chairs. Eben Rosenthal, MD (Stanford University; CA), who was president of ASIGS at the time of the acquisition and who co-founded that group, maintains a chair position in the OSN-IG, with a dedicated focus on ASIGS.
Now, the three OSN chairs have coauthored, along with two additional Stanford professors, a new paper that reviews the optical approaches under clinical investigation—including various fluorescent agents, and the specific hardware systems supporting them—and discusses emerging strategies.1
In pursuit of FDA approval
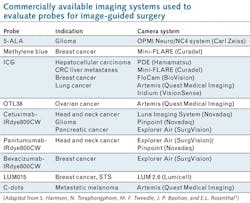
The authors report the development and testing of many new probes in recent years—and the creation and application of an equal number of fluorescence camera systems to clinically evaluate these agents (see table). And while they expect such approaches to enable greater precision in tumor surgeries, with resulting improvement in functional and oncologic outcomes for patients, they state regulatory approval is unclear because no approved agent exists in the U.S.
They warn that clinical trials will need to be carefully designed to show patient benefit—without huge numbers of participants or long-term endpoints). So, the OSN plans to engage regulators in active dialog.
In fact, OSN was founded on the idea of "a strategy to place the field out ahead of regulatory requirements such that we inform the FDA regulatory process," according to Tweedle and Basilion. The group has articulated support for experimentation to resolve standardization issues among its key activities, along with publication of papers defining requirements for hardware and surgical reagent development and production. "We believe that the FDA will look to experts in the field to help define this 'new surgical era' and we will already have developed a cogent plan," they stated.
REFERENCE
1. S. Harmsen, N. Teraphongphom, M. F. Tweedle, J. P. Basilion, and E. L. Rosenthal, Mol. Imaging Biol., doi:10.1007/s11307-017-1054-1 (2017).

Barbara Gefvert | Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.