Photoacoustics/Microscopy/Bioimaging: Photoacoustic microscopy enters the mainstream
DEPENG WANG, RACHEL LIM, JUN XIA, and YANG PU
Near-infrared (near-IR) optical spectroscopy and imaging are powerful tools, enabling noninvasive screening and clinical diagnosis of diseased tissues—not only on the surface of a body, but also below the surface, even reaching organs located deep within.1
Spectroscopic techniques enable quantitative investigation of tissue absorption and scattering properties, and in vivo differentiation of normal and cancerous tissues using markers such as water content, oxygen saturation level, and hemoglobin concentration.
Although near-IR light can reach several centimeters into tissue, by the time a photon reaches such depth, it will likely have undergone hundreds of scattering events. A scrambled photon path inhibits effective optical focusing. Fortunately, photons in tissue can induce ultrasonic waves, which are scattered much less.
As a hybrid technique that forms images by detecting induced pressure waves, photoacoustic tomography (PAT) breaks the optical diffusion limit by capitalizing on low acoustic scattering in tissue.2 PAT can thus outperform diffuse optical tomography (DOT), and allows multiscale high-resolution imaging of biological structures ranging in size from organelles to organs.3
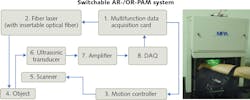
One of the two major state-of-the-art implementations of PAT are photoacoustic computed tomography (PACT) and focused-scanning photoacoustic microscopy (PAM). The latter is further classified into optical-resolution (OR-PAM) and acoustic-resolution (AR-PAM) variants that give the finer optical or ultrasonic focuses. Regardless of the implementation, PAM can scale resolution laterally from subwavelength to a few wavelengths by varying the numerical aperture (NA) according to the different imaging depths.
The system and its lasers
Photoacoustic microscopy has now entered a new era of development, having just recently achieved system commercialization. Now, PAM is helping to advance life science research in neuroscience, cell biology, and in vivo imaging.
At this early stage, there is just one manufacturer of PAM systems: MicroPhotoAcoustics (MPA; Ronkonkoma, NY) is a spinoff of Advanced Optowave, which was founded in 2010 by the inventors of three-dimensional PAM. MPA has developed a commercial PAM system with switchable optical and acoustic resolution (OR- and AR-PAM), using multiple patents licensed from the lab of Lihong Wang, who pioneered photoacoustics at Washington University in St. Louis (St. Louis, MO) before moving to the California Institute of Technology (Pasadena, CA).
The system includes different excitation sources for the different modes. The sources for OR-PAM are two kilohertz-tunable, Q-switched, fiber-based solid-state lasers offering a 5 kHz pulse repetition rate and 9 ns pulse duration with 532 and 559 nm,4 respectively, and output to achieve functional photoacoustic tomography for sO2 (oxygen saturation of hemoglobin) imaging. The excitation source for AR-PAM is a Ti:sapphire laser system with a selection of up to eight output wavelengths, in the range from 700 to 900 nm to achieve deep-tissue imaging. Switching between OR- and AR-PAM is a matter of replacing a single-mode fiber with a multimode fiber configured with a different collimator lens.
In OR-PAM mode, the system provides up to 1 mm penetration depth and resolutions of 5 μm lateral and 30 μm axial, while AR-PAM offers up to 3 mm imaging depth and resolutions of 45 μm lateral and 30 μm axial. Both modes use raster scan to form a 3D image. The scanning step sizes for OR- and AR-PAM are 0.625 and 6.25 μm, respectively. Therefore, to image the same region, the imaging frame rate for AR-PAM is 10X that of OR-PAM (see Fig. 2).Applications
Researchers have used the system for a range of applications, including preclinical neural imaging; imaging of cell nuclei in intestine, ear, and leg; and clinical human imaging of finger cuticle.
Their work has explored the use of alternative light sources, filters, and algorithms to improve the capabilities of PAM, and is promising new applications. These efforts have enabled MPA to further develop the technology to meet the needs of life sciences.
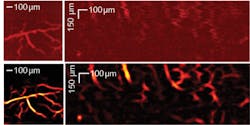
Researchers in Japan used the instrument in OR-PAM mode for vascular imaging, and then used a vessel filter to process the PAM image to achieve high-sensitivity output.5 Figure 1 shows original and filtered vascular images of a living mouse brain and human finger cuticle. Even small vessels that are otherwise difficult to discern from the background are distinct with the filter, hinting that PAM could be a valuable tool for diagnosis of vascular-related disease.
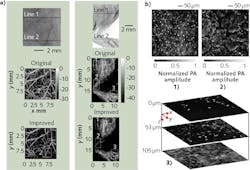
Using the same system, researchers in South Korea demonstrated the use of an improved image reconstruction algorithm to produce high-resolution AR-PAM imagery. Figure 3a presents photographs of mouse ear and leg, and corresponding AR-PAM images reconstructed with conventional and improved algorithms.6 Because the new algorithm improves resolution in the out-of-focus regions, it promises to greatly extend the system's depth of view.The system has also been used to explore new applications. For instance, researchers (at Washington University in St. Louis and the University of Southern California) transformed the instrument into a UV-PAM setup by replacing the excitation laser with a UV light source. This allowed them to use the setup for label-free imaging of cell nuclei, which strongly absorb UV light (see Fig. 3b).7
The commercialization of photoacoustic microscopy has already facilitated progress in biomedical imaging, and more progress is on the way. With the continuation of new technological advancements and discoveries, MPA plans to further advance PAM to achieve faster imaging speed, higher spatial resolution at deeper tissue layer, and address a broader range of biomedical applications.
ACKNOWLEDGEMENT
At press time, MPA announced the release of its second-generation system, which includes a waterproof microelectromechanical systems (MEMS) scanner—designed in collaboration with Pohang University of Science and Technology (South Korea)—that promises to dramatically increase the system's imaging speed to ~1000 A-lines (i.e., depth-resolvable 1D profiles) per second.
REFERENCES
1. Y. Pu et al., Appl. Opt., 53, 11, 2345–2351 (2014).
2. L. V. Wang and S. Hu, Science, 335, 6075, 1458–1462 (2012).
3. L. V. Wang, Nat. Photon., 3, 9, 503–509 (2009).
4. L. V. Wang and J. Yao, Nat. Methods, 13, 8, 627–637 (Aug. 2016).
5. I. U. Haq, R. Nagoaka, T. Makino, T. Tabata, and Y. Saijo, "3D Gabor wavelet based vessel filtering of photoacoustic images," Proc. IEEE Eng. Med. Biol. Soc. 2016, 3883–3886 (Aug. 2016); doi:10.1109/embc.2016.7591576.
6. J. Park et al., J. Biomed. Opt., 21, 3, 036010–036010 (2016).
7. D.-K. Yao, K. Maslov, K. K. Shung, Q. Zhou, and L. V. Wang, Opt. Lett., 35, 24, 4139-4141 (2010).
Depeng Wang is a senior PhD student, Rachel Lim is a research assistant, and Jun Xia is an assistant professor in the Department of Biomedical Engineering, all at the State University of New York at Buffalo, Buffalo, NY; www.acsu.buffalo.edu/~junxia, while Yang Pu is a senior scientist at MicroPhotoAcoustics, Ronkonkoma, NY; e-mail: [email protected]; http://biompa.com.