3D OPTICAL IMAGING: Holoscopy makes ultrafast lensless imaging of scattering tissues possible
GEREON HÜTTMANN, GESA FRANKE, CHRISTIAN LÜHRS, PETER KOCH, and DIERCK HILLMANN
Advances in optical imaging have changed biomedicine, from the invention of the optical microscope to biochips, flow cytometry, and the latest versions of nonlinear microscopy. But there is still much to be understood. Biology is inherently a three-dimensional (3D) science, with parallel interactions between macromolecules, cell organelles, and cells. These massively parallel dynamic biological interactions, which have time constants down to the millisecond range, are the basis of the enormous complexity of life and require fast, noninvasive 3D imaging modalities for proper study. Unfortunately, present microscopic techniques that rely on serial scanning of tissue volumes are often too slow.
In confocal imaging and nonlinear microscopy, the threshold of the tissue damage and photophysical/photochemical saturation effects limit the excitation flux and the maximal photon rate from a voxel. Confocal serial scanning is a bottleneck in increasing the imaging speed.
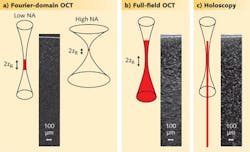
One of the fastest optical imaging modalities today is Fourier-domain OCT (FD-OCT), which uses interference at multiple wavelengths to determine the depths of the different scattering structures. The intense scattering signal and the parallel detection of photons from all depths facilitate an imaging speed that can reach gigavoxels per second. However, the parallel detection is only possible within the beam waist of the source due to the confocal detection scheme (see Fig. 1). For a numerical aperture (NA) of 0.05, an OCT image of iron-oxide nanoparticles embedded in a transparent medium only reveals particles in a small region around the focal plane. Imaging the entire volume requires serial scanning at different depths. Higher NAs to increase resolution and the number of collected photons unfortunately results in a further decreased depth of focus.
For ultimate imaging speed a twofold parallelization of the imaging process is needed. The tissue should be irradiated by an extended beam to avoid high irradiance at the focal point ,and all returning photons should be detected at high NA and allocated to their origin in parallel, irrespective of the depth from which they originate. Fourier-domain full-field OCT, which uses a tunable light source and camera for parallel OCT imaging without confocal scanning, has parallel illumination and detects light from all depths, but gives only blurred images outside the focal plane.1
But finally, high-resolution imaging over the whole depth is possible by combining Fourier-domain full-field, swept-source OCT with digital holography (DH), which is not limited to imaging a certain plane but can record a whole volume with diffraction-limited resolution (see table). Combining both methods into holoscopy enables detection of all the scattered photons and can also be used to trace back their origin with high lateral and axial resolution. Using holoscopy, the imaging speed increase can be estimated by the ratio of the desired depth of field (DOF) to twice the Rayleigh length (2zR), which is the depth region from which sharp conventional imaging is possible. At 0.8 NA and 100 μm DOF, holoscopy replaces 200 images in different planes with one volumetric image.Holoscopy emerges
The ideas that contributed to holoscopy can be traced back more than 60 years. In 1949 Dennis Gabor proposed holography—using interference with a separate reference wave to record and retrieve the full information from a scattered light field.2 Emil Wolf showed at the end of the 1960s that light from not-too-strongly scattering volumes contains the full information on the inner scattering structure.3,4 An experimental demonstration of this idea was published by Fercher et al. at the end at the 1970s.5 However, due to the lack of digital recording media and computational power, this work was not of practical value.
Both Wolf and Fercher reported that it is not sufficient to record one hologram to image a full volume because the electromagnetic field in the 2D recording plane is not determined in a unique way by a 3D arrangement of scattering centers; that is, one degree of freedom is missing. If single digital holograms of scattering volumes are reconstructed, effectively the sharp image in every single plane is overlaid and obscured by blurred images of other planes. Illuminations from different directions or recordings of multiple holograms at different wavelengths must provide the missing information.6
From this pioneering work, it was clear that holography at different wavelengths should be able to image scattering tissues similar to OCT. However, until recently, technology was not available for holographic tissue imaging. But with the advent of fast computers and fast, high-resolution cameras, a digital version of holography became increasingly popular, especially for microscopic imaging.7
Digital holography replaces the optical reconstruction of a hologram by numerical computation. After the recording of the interference pattern by the camera, the reconstruction (retrieval of the object information) is done mathematically by propagating the recorded light field back to the sample volume. While DH successfully images 3D surfaces and thin microscopic samples, it can only image scattering tissues if depth information is obtained by recording holograms at multiple wavelengths. Digital holography at different wavelengths has been experimentally demonstrated by various research groups, but the achieved image quality has been limited.8-11
The key to practical holoscopy, which images scattering volumes over several tens of Rayleigh lengths, is the consequent integration of swept-source OCT with DH.
Ultrafast and lensless
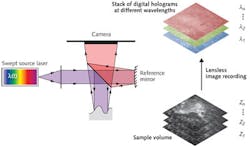
In a first series of successful experiments at the University of Lübeck, a Michelson interferometer was used with a semiconductor-laser-based tunable light source and a monochromatic CMOS camera with a 1536 × 1536 pixel imager (see Fig. 2). A total of 1024 images were taken from a sample with scattering nanoparticles at different wavelengths. For the reconstruction of the object information, the light field was calculated from each hologram by the angular spectrum approach in a certain plane of the volume. Subsequently, by a Fourier transform with respect to wavenumber, the depth information was obtained at each pixel.Using this one-step method, the acquisition time for the complete volume with 330 megavoxels was only 1.2 s. This turned out to be too slow for in vivo imaging because tissue motion within the acquisition time leads to blurring of the axial structures. However, increasing the image-acquisition rate by using an ultrahigh-speed Photron (San Diego, CA) Fastcam SA5 CMOS camera with 7000 frames/s made in vivo imaging of skin at 3.5 gigavoxels per second possible.
By combining OCT with digital holography into holoscopy, we can image scattering tissues, ex vivo and in vivo, over a depth of more than 30 Rayleigh lengths with OCT-like quality. And since holoscopy uses parallel illumination and detects all the scattered photons in a volume within a potentially large acceptance angle, it is not limited by the depth of field as in FD-OCT. Our next step is to achieve micrometer-resolution imaging in the lateral and axial direction by further increasing the NA through additional optics and using a Ti:sapphire laser with a >200 nm spectral range that has the instantaneous bandwidth and phase stability needed for holoscopy.12
REFERENCES
1. T. Bonin et al., Opt. Lett., 35, 20, 3432–3434 (2010).
2. D. Gabor, Nature, 161, 4098, 777–778 (1948).
3. E. Wolf, Opt. Comm., 1, 4, 153–156 (1969).
4. E. Wolf, J. Opt. Soc. Am., 60, 1, 18–20 (1970).
5. A.F. Fercher et al., Appl. Opt., 18, 14, 2427–2439 (1979).
6. A.F. Fercher, Zeitschrift für Medizinische Physik, 20, 4, 251–276 (2010).
7. M.K. Kim, SPIE Reviews, 1, 018005 (2010).
8. P. Blazkiewicz et al., Appl. Opt., 44, 36, 7722–7729 (2005).
9. M.C. Potcoava and M.K. Kim, Measurement Sci. and Technol., 19, 7, 074010 (2008).
10. D.V. Shabanov et al., Laser Phys. Lett., 6, 10, 753–758 (2009).
11. V.S. Dmitry et al., Digital Holography and Three-Dimensional Imaging (DH) conference, paper DMC7, Miami, FL (April 2010).
12. G.L. Franke et al., Photonics West BiOS 2012, paper 8213-75, San Francisco, CA (Jan. 25, 2012).
Gereon Hüttmann is a senior scientist and Gesa Franke is a PhD student at the Institute for Biomedical Optics at the University of Lübeck, Peter-Monnik-Weg 4, 23562 Lübeck, Germany; e-mail: [email protected]; www.bmo.uni-luebeck.de. Christian Lührs and Peter Koch are product development engineers and Dierck Hillmann is a software engineer at Thorlabs GmbH, Maria-Goeppert-Strasse 1, 23562 Lübeck, Germany.