Genetically engineered bioluminscence enables optical imaging of the spread of disease, the effectiveness of drug therapies, and the expression of genetic traits inside of living animals.
A wide variety of new technologies have combined to form a revolution in the imaging of cellular and molecular processes inside living animals (see "Biomedical Optical Imaging" supplement, Laser Focus World, March 2004) These new technologies allow researchers to follow, almost in real time, the step-by-step invasion of a disease into tissue cells or how a gene is "expressed" by producing proteins in different parts of the body. This ability is likely to facilitate the development of new drug and gene therapies to combat cancer and other previously intractable diseases.
One category of these new techniques extends the abilities of standard diagnostic imaging systems, such as magnetic resonance imaging (MRI) and positron emission tomography (PET), enabling them to capture some of these processes. However, while they excel at creating high-resolution three-dimensional anatomical images, these systems are complex, slow, and expensive. A second class of new imaging technologies relies on inducing the cells under study to glow with their own biologically produced light. While lower in resolution, these less complicated systems produce images faster and possess their own unique capabilities.
The genetic report
About a decade ago, researchers began genetically engineering animal cells to add so-called "reporter" genes, which are connected to target genes whose activity they wish to study. One type of reporter gene causes a dye to change color if it enters a cell in which the reporter gene has become active. Because the reporter and target genes become active together, the newly colored dye indicates the presence of the protein expression of the target gene.
In these experiments, however, either the cells are studied outside of the animal (in vitro) or some of the test animals are killed at regular time intervals to examine the progress of the dye. Recently, however, it has become possible to study the disease processes in living animals by using reporter genes that actually cause selected cells to glow from within. Such genes can either produce fluorescence or mimic the activity of organisms that generate their own light, which is called "bioluminescence."
null
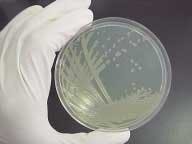
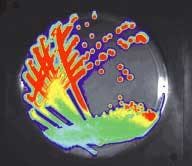
FIGURE 1. The bacteria P. aeruginosa has been genetically engineered for bioluminescence (top). Special imaging systems can record the genetic activity of the living organism (bottom).
Bioluminescence has an advantage over fluorescent methods in that the cells of interest illuminate without an external light source, and produce a lower level of background noise. A surprising variety of organisms produce their own light (see Fig. 1). Fireflies, for example, possess the luc gene responsible for the production of the enzyme luciferase. In the presence of oxygen, ATP, and a biological substrate called luciferin, luciferase produces the firefly's ghostly green glow at a wavelength around 560 nm.
Glowing mice
The luc gene is used as a reporter of cellular activity in two general ways: either added to the DNA of disease-producing cells or to the DNA of a test animal. Spliced into the DNA of disease cells, including bacteria and tumor cells, it enables the spread of the disease to be tracked when these cells are injected into an animal. In this case, when the luciferin substrate is also injected, the tissue invaded by the luciferase-producing disease illuminates.
The light emitted is proportional to the number of disease cells that have infected the local tissue. The spread of the disease can be compared in animals that have been treated with an experimental drug versus the infection in control animals. In comparison to conventional studies, many fewer animals are used, and many fewer killed, through the use of bioluminescent reporters. In addition, the results are more reliable because the same individual animals are studied throughout the experiment.
The other general method of application is to introduce the reporter gene into the embryo of a test animal, most commonly a mouse, to study a particular trait. The trait gene is identified and the luc gene spliced next to it. As the mouse matures, most of its cells will develop with the ability to produce light. When a drug mixed with the substrate is injected into the mouse, the cells where the mixture arrives make luciferase, and thus the tissues receiving the drug glow with visible light. This process is useful to determine in which tissues the specific genes are active and how drugs alter their activity.
Looking right through you
Mammalian tissue scatters and absorbs visible light. Hemoglobin has a strong blue-green absorption, but is somewhat transparent for wavelengths longer than 600 nm. A tissue sample 1 cm deep will attenuate 550-nm light down to about 10-10, while a 650-nm signal will be attenuated to a more reasonable 10-2 level.
In comparison to absorption, an effective scattering length in tissue might be 0.25 cm, or even as short as 0.05 cm in the red and near-IR spectrum. This scattering results from changes in the refractive index at membrane interfaces. Thus, while it is possible to transmit a weak red light from the inside of a mammal, the photonic signal will be very diffuse.
Although it peaks at about 560 nm, the luciferase reaction has a broad emission that extends well into the red. The number of photons emitted by this reaction varies greatly with the details of the biology, but it appears to be in the range of 5 to 100 photons per cell per second for cells of interest.1 Just enough light is transmitted through a few centimeters of tissue to reconstruct an image of the emitting cells (but not enough to be seen with the naked eye).
A model image
To form an image, the very low signal levels require that system noise be kept to a bare minimum, which complicates detector selection. Image-intensified CCD cameras allow individual photons to be counted with very little noise, but have low sensitivity in the red and near-IR. Integrating CCD arrays have high sensitivity, but require cryogenic cooling to reduce the relatively high dark current.
In all cases, noise from the readout electronics must be kept low. The signal can be increased relative to the electronic noise by grouping pixel signals together to effectively form a larger pixel with the same readout circuitry. Resolution suffers, of course, but for in vivo studies the diffuse images do not benefit from higher spatial resolution.
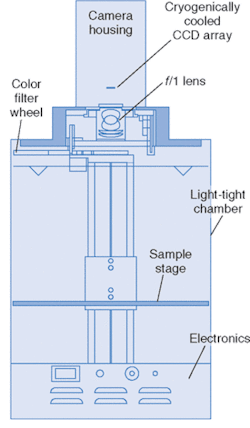
FIGURE 2. An example of a commercial bioluminescent-imaging system is less than a meter in height.
This technology has developed to the point where it is commercially available. The diagram of one such system, called IVIS, from Xenogen (Alameda, CA) is pictured in Fig. 2. The anesthetized animal is placed on a stage in a chamber that eliminates background light, and the stage is heated to keep the animal warm. The stage translates vertically to change the field of view.
The cooled, back-illuminated, integrated CCD array detects the light captured by the 50-mm ƒ/1 lens focused on the surface of the animal. A conventional camera takes a black and white photo of the animal, and then the bioluminescent image is captured. Imaging times are typically between 30 seconds and 5 minutes. The false-color images, superimposed over the standard photo, represent the number of photons emitted per unit area (see Fig. 3).
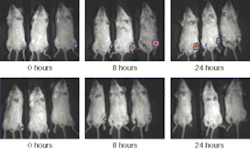
FIGURE 3. These two sets of images show the results at three time points for anesthetized mice infected with S. aureus, which has been tagged with a bioluminescent reporter. The mice in the bottom images have been treated with amoxicillin.
To reconstruct an image through the strong scattering of the tissue requires sophisticated modeling and statistical analysis. Monte Carlo representations of diffusion have created realistic results. Different models can be used to anticipate scattering caused by different kinds of tissue (lungs, muscle, and so on).
For tissue with a scattering length of 0.25 cm, and assuming an emission of 30 photons per cell per second, the IVIS system can require 104 to 105 illuminated cells to image processes in deep organs at 1 cm, while subcutaneous study can require as few as 500 cells. These results depend strongly on the experimental details. The use of color filters can reveal additional information about the depth and distribution of the illuminated cells.
A hidden infection exposed
Applications of bioluminescent imaging are yielding practical results. One example is a study of the Listeria germ that is responsible for about 500 food-poisoning deaths annually in the United States, many of them pregnant women. About 20% to 40% of those affected die even after antibiotic treatment.
A team lead by Christopher Contag, a professor at Stanford University (Stanford CA) and a pioneer in the field of bioluminescent imaging, tagged the germ with the luc gene and injected it into mice.2 The luciferase reaction unexpectedly revealed that Listeria can reside in the gall bladder as well as in the liver. What's more, Listeria was found to reproduce outside the host's cells altogether, leaving open the possibility that apparently healthy food workers can spread the organism. It is hoped that these findings will lead to a greater sense of urgency to public health practices.
New developments
In summary, bioluminescent imagers provide pictorial information about molecular activity within cells. It can track both the location and development of a disease, the effectiveness of an experimental drug, the activity of a gene, and so on. On the practical side, bioluminescent imagers are lower in cost than diagnostic systems (PET scanners, for example, use cyclotron radiation), are simpler to operate, and can capture images from several animals at once.
The ability of bioluminescent imagers to resolve structures, including tumors, is limited when compared to diagnostic systems. Newer bioluminescent imagers can locate a light-emitting collection of cells to within 1 to 3 mm, depending on their depth in tissue, but at the expense of longer exposure times. Development is under way to capture images from multiple angles to produce three-dimensional bioluminescent pictures in a manner similar to computed tomography.
REFERENCES
- J. Biomedical Optics 6(4) 432 (October 2001).
- Science 303(5661) 851 (Feb. 6, 2004).