DETECTORS FOR MICROSCOPY: Next-generation 3-D detector improves single-molecule imaging
XAVIER MICHALET, KATSUSHI ARISAKA, OSWALD H.W. SIEGMUND, JOHN V. VALLERGA, PATRICK JELINSKY, and JACQUES E. MILLAUD
The exquisite sensitivity of light detectors used in wide-field fluorescence microscopy or scanning confocal microscopy has recently allowed the detection, tracking, and spectroscopic analysis of the fluorescence emission of single molecules.1, 2 Single-fluorescence emitters such as dye molecules or quantum dots have been used to tag proteins, DNA, or RNA, and monitor their location or interaction with other molecules in vitro or in vivo.3 Single-molecule techniques can reveal rare events, discrete steps, or the complete spectrum of static and dynamic properties that are otherwise hidden in ensemble measurement—in short, they have the potential to revolutionize our understanding of how biomolecules actually work and interact in complex molecular networks.
However, single-molecule spectroscopy (SMS) currently suffers from specific detector limitations. Wide-field detectors (charge-coupled devices, including intensified CCDs or electron-multiplying CCDs), which allow the study of hundreds of single molecules at once, have poor time resolution. Time-gated cameras, which have picosecond timing capability, are very photon-inefficient detectors not suited to the study of single molecules or rapidly changing samples. Point-like single-photon-counting detectors (photomultiplier tubes or single-photon avalanche photodiodes) with subnanosecond time resolution require scanning to form an image and are therefore inefficient imaging detectors, limiting their use to the study of isolated, static molecules, or molecules diffusing through a fixed femtoliter volume.
The ideal SMS detector
Motivated by the demanding needs of single-molecule fluorescence spectroscopy and imaging applications, our team of researchers from the University of California at Los Angeles (UCLA; Los Angeles, CA), the University of California at Berkeley (Berkeley, CA), and Lawrence Livermore National Laboratory (LLNL; Livermore, CA) started a program in detector development. An ideal SMS detector should combine the properties of both types of detectors and allow simultaneous observation of hundreds of single molecules emitting a detected signal of up to 100 kHz (a 100 kHz maximum local count rate) and a 50 MHz maximum global count rate.
Detectors combining spatial and temporal capabilities have been commercialized and used in the recent past, but they are far from approaching these ideal specifications. They all use a similar design based on a multialkali photocathode with quantum efficiency (QE) less than 20% in the visible spectrum. The photocathode is followed by one or more electron-multiplying microchannel plates (MCPs) generating a cloud of several millions of electrons at the back of the MCP. These electrons are proximity-focused onto and collected by a plain resistive anode or quadrant-capacitive anode that allows computing the position of the center of mass of the cloud. The readout electronics of this type of anode limits the acquisition rate to less than 100 kHz.
To attain higher global count rates needed for wide-field observation of multiple single molecules and rapidly changing fluorescent samples, a faster type of position-sensitive anode is needed. Based on the work of the Space Sciences Laboratory at UC Berkeley, we designed and constructed a high-throughput three-dimensional detector (H33D) using a cross-delay anode that allows a maximum readout rate of approximately 700 kHz.4
H33D description
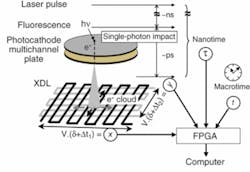
To fabricate the H33D, an S20 multi-alkali photocathode was deposited on a fused-silica window and proximity-focused on a microchannel-plate z stack (see Fig. 1).5-7 A 30 × 30 mm cross-delay line was vacuum sealed approximately 6 mm behind this assembly. The role of the photocathode is to convert each incoming fluorescence photon (or at least a fractional QE of them) into a photoelectron. Each photoelectron generates a cloud of secondary electrons at the back of the MCP, which spreads and impacts the two separate and orthogonal zigzag patterns of the delay lines. Charge propagation through the delay lines results in detectable pulses at both ends of each delay line, the temporal separation of which gives access to the position along each delay line (x and y).
The precise timing (nanotime, τ) of each detected photon with respect to the exciting laser pulse is obtained with a commercial time-to-digital converter (TDC), which measures the separation of each voltage pulse generated at the back of the microchannel plate (Start) from the laser pulse signal (Stop). A field-programmable gate array (FPGA) associates the (x, y, τ) measurements to a time-stamp (macrotime, T) defined as the number of laser pulses since the beginning of the experiment. Each (x, y, τ, T) data set is then asynchronously transferred to a computer via a fast-digital-interface board. Software written in LabView performs data storage, online image visualization, and data analysis.
H33D performance
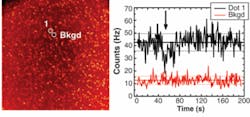
The measured QE of our S20 photocathode decreases from 18% at 400 nm to 8.5% at 520 nm and 3% at 630 nm—typical values for this type of material, but significantly lower than standard detectors used in single-molecule spectroscopy. Imaging performance, as measured with test samples (reticle and/or subdiffraction fluorescent beads) shows a spatial resolution better than 100 µm at the microchannel-plate gain of approximately 107 used, varying very little with the local count rate. Minimal spatial nonlinearities at the rim of the detection area are easily corrected by software. The temporal resolution measured using an attenuated pulsed diode laser with a pulse width of 80 ps and a repetition rate of 10 kHz is better than 100 ps full width at half maximum.
Applications and results
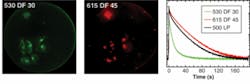
To demonstrate the basic capabilities of the H33D in imaging fluorescent samples while simultaneously providing high-resolution temporal information on fluorescence lifetime, samples of dyes with known lifetimes were imaged as thin slabs (approximately 30 µm) of liquid between two glass coverslips using a femtosecond Ti:sapphire laser tuned at 720 nm and frequency doubled using a BBO crystal. By plotting a histogram of the nanotimes of all recorded photons, a reversed fluorescence decay curve convolved with the response function of the instruments is obtained. Perfect single exponential fits of the decay curves were obtained for fluorescent dyes such as Rhodamine 6G and ethidium bromide, recovering their known fluorescence lifetime without further deconvolution. For quantum dots, a more complex decay curve was observed, necessitating a stretched exponential fit, in agreement with published data.8Next-generation perspectives
Although the H33D can be used as a static imager by representing the intensity per pixel during a fixed period of time, the integration time can be adjusted at will by the user post-acquisition, allowing the creation of image sequences or “movies” with arbitrary time resolution and limited only by the available signal-to-background ratio.
The current H33D prototype still suffers from a relatively low QE and a limited maximum global counting rate. Recent developments in fast gallium arsenide (GaAs) or GaAs phosphide (GaAsP) photocathodes could offer significant QE improvements in future detector generations. The current limitation on global counting rate is due to the anode readout electronic speed. While improvements in speed can be obtained, a fundamental limit in local and global count rates is set by the high microchannel-plate gain currently used. For specific applications, alternative readout schemes for this type of photon-counting device are possible.9, 10 Despite these technical challenges, future H33D detectors that use better photocathode and readout schemes will further improve temporally and spatially resolved spectroscopy and microscopy at the single-molecule level.
ACKNOWLEDGMENTS
This work was supported by NIH grant 5R21 RR017474 and NSF grant DBI-0552099. We thank Fabien Pinaud (UCLA) for providing the HeLa cells shown in Fig. 3.
REFERENCES:
1. S. Weiss, Science 283, 1676 (1999).
2. X. Michalet et al., J. Modern Optics 54 (2007).
3. X. Michalet et al., Science 307, 538 (2005).
4. O. H. W. Siegmund et al., Proc. SPIE 2280, 89 (1994)
5. O. H. W. Siegmund et al., IEEE Nuclear Symposium Conf. Record N14-55, 448 (2005).
6. X. Michalet et al., Nucl. Instr. Meth. Phys. Res. A 567, 133 (2006).
7. X. Michalet et al., Proc. SPIE 6092, 60920M (2006).
8. X. Michalet et al., Proc. SPIE 6372, 63720E (2006).
9. A. S. Tremsin et al., IEEE Trans. Nucl. Sci. 51, 1707 (2004).
10. T. Ohnukia, Proc. SPIE 6092, 60920P (2006).
Xavier Michalet is a scientist and Shimon Weiss is a professor in the Dept. of Chemistry and Katsushi Arisaka is a professor in the Dept. of Physics at UCLA, Los Angeles, CA, 90095; Oswald H.W. Siegmund is a professor and John V. Vallerga and Patrick Jelinsky are research physicists at the Space Sciences Laboratory at the UC Berkeley, 7 Gauss Way, Berkeley, CA 94720; Jacques E. Millaud recently retired from Lawrence Livermore National Laboratory, 7000 East Ave., Livermore, CA 94550; e-mail: [email protected].