The detection of rare or short-duration events often uses a light-based system because of the availability of very sensitive light detectors such as photomultipliers (PMTs). These devices convert the optical photons to an electrical signal and then amplify it as much as a million times. While most amplifiers add some noise to the signal, PMT amplification is almost noise free. The resulting gain makes it possible to detect single photons. In addition, the amplifier's bandwidth of several gigahertz facilitates detection of signals that change rapidly or last for only a brief time.
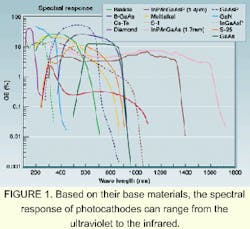
A PMT also can be sensitive in the ultraviolet or the infrared. Its photocathode converts the photon into an electron by the photoelectric effect. This active area can be made from a large number of different materials to tailor the spectral response (see Fig. 1). Unlike other detectors, fabrication of PMTs with large active areas is relatively easy and cost-effective. The largest PMT is half a meter in diameter, while the smallest fits into a small can.
These features have made the PMT popular in such demanding applications as medical imaging, gene sequencing, and high-energy physics.
Neutrino experiments
One of the largest PMT active areas is a unit 0.5 m in diameter designed for the Kamiokande neutrino experiment. Japan's Super Kamiokande Observatory is buried about a kilometer below the earth's surface. The detector is a cylinder 41.4 m high and 39.3 m in diameter. Inside is an outer tank holding 18,000 tons of pure water (to filter out cosmic-ray muons and radiation from the surrounding rock), as well as an inner tank with 32,000 tons of water. An array of 11,146 PMTs surrounds the inner tank (see Fig. 2).Neutrinos having no charge cannot be detected directly, so the Super Kamiokande experiment detects charged particles scattered by the neutrinos. If a charged particle travels at >75% of the speed of light, it produces a cone of blue light called Cherenkov light that is detectable by the array of PMTs.
The type of neutrino involved in the collision determines which particle creates the Cherenkov light. From the pattern of light and its arrival time at each detector, it is possible to determine the direction of a scattered particle and whether it is a muon or electron. Analysis of the types of particles detected has led the Kamiokande researchers to conclude that the neutrino has a mass—a discovery that should fuel a reevaluation of how the universe is constructed.
Medical detection
PMTs also play a key role in nuclear imaging. For instance, the gamma camera, the single-photon-emission computed tomography (SPECT) camera, and the positron-emission camera (PET) all use PMTs to quickly and efficiently detect single gamma ray photons. The PMTs do not detect the gamma rays directly. Instead, a scintillator crystal interacts with the gamma ray producing between 20 and 40 visible photons for each gamma photon (see Fig. 3). The application and construction of the three cameras is different, so this article will discuss only the PET camera.When a radioactive nucleotide such as F17, C11, or O15 decays, it produces a positron, which then quickly reacts with an electron. Subsequent annihilation of the particles produces two gamma rays that travel at almost 180° with respect to each other. By detecting the two gamma rays with a pair of crystals and PMTs in coincidence, background signals from environmental radiation can be eliminated. A ring of such detectors can detect gamma rays incident from many directions. Reconstruction software then can produce a three-dimensional image of the radioactive tracers.
The strength of the technique lies in the capability to incorporate the three different nucleotide traces into compounds such as deoxyfluoroglucose, water, and organic chemicals. The deoxyfluoroglucose, for instance, can be injected into the body of a patient. Those cells that are metabolizing rapidly, such as cancer cells, will accumulate more F17 and thus appear brighter in the PET image. This technique is used to detect cancer metastasis.
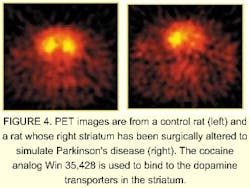
In contrast, a reduction in F17 can help researchers spot deterioration due to dementia. Small pharmacological agents that bind to specific chemical receptors in the brain can be used to study disease models and how proposed treatments will affect test animals (see Fig. 4). For example, researchers can study changes in the uptake of L-dopa in Parkinson's disease, or the increase in the number of dopamine D2 receptors due to cocaine use without sacrificing the animal.Optical methods also can help researchers detect disease organisms. Two approaches are under study to detect the presence of a contaminant in the air and then to identify the contamination in real time. To be useful, these approaches must ultimately be portable. The first method uses mass spectroscopy, the second uses laser-induced fluorescence.
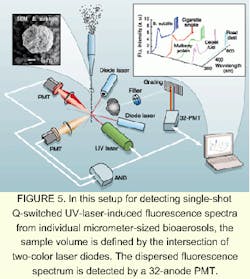
Working with researchers at the US Army Research Laboratory, Adelphi, MD, Yong-Le Pan and Richard Chang at Yale University (New Haven, CT) are pursuing the laser-induced fluorescence approach. Their goal is to distinguish between bioaerosols and non-bioaerosols, in this case by using a Q-switched UV laser to excite the fluorescence (see Fig. 5).For the equipment to filter out similar spectra from much smaller atmospheric contaminants, such as aromatic hydrocarbons, the laser must be triggered only when a particle 1 to 50 µm in radius is present in the sampling volume. Two laser diodes emitting at 635 and 670 nm, respectively, are crossed and focused to define a small detection volume. If a particle enters this detection volume, the light from the laser diodes is scattered into two PMTs with neutral density filters and interference filters. If the pulse from each PMT exceeds a preset threshold, but is smaller than a maximum value, a signal is sent to an AND gate. The purpose of the discriminator is to determine that a particle of specific size has entered the sampling volume.
When the signals from both PMTs overlap for more than 3 ns, a trigger pulse is sent to the Q-switched UV laser and the detector. Either 266- or 351-nm light is used to excite the micron-size particle in the detection volume. The light is imaged onto a spectrograph, which either has an intensified charge-coupled-device (ICCD) camera or a 32- channel PMT in its image plane. The PMT has much lower resolution than the ICCD. For biologically relevant compounds, the emission is broad and the 32-channel PMT provides sufficient spectral resolution in the range of 300 to 800 nm. In addition, it has much higher sample throughput.
Both systems can detect fluorescence emission spectra from individual particles and distinguish between bacteria and some nonbiological aerosols. Neither technique is yet capable of identifying the bacteria.
Under the microscope
Detailed study of individual bacteria or other cells still requires a microscope. Conventional microscopes, however, present problems for researchers studying a cell in three dimensions because of scattered light from planes outside the image plane.
An alternative is a confocal microscope, which focuses the excitation laser to a spot in the image plane. The laser excites a dye, which fluoresces. This fluorescence is imaged onto an aperture, which rejects light that does not originate in the image plane and passes light that does. This is then detected by a PMT.
Scanning both the excitation and the emission channels with mirrors generates the two-dimensional image. Images from different planes are obtained by changing the height of the focus within the sample while also changing the height of the detection plane. To prevent dye photobleaching, the laser beam is attenuated as much as possible. Thus, at each pixel there may be only 10 to 100 photons that strike the detector. Their ability to count single photons makes PMTs useful here.
The confocal geometry also works with gene chips. Using lithographic or ink-jet printing, arrays of polymers of DNA base pairs called oligomers are placed on a surface such as a glass slide. Each oligomer has a unique sequence of bases. The number of unique sites can range up to hundreds of thousands. An unknown sample of DNA is first copied using the polymerase chain reaction (PCR). The bases used for the PCR are labeled with fluorescent tags. The copies are added to a chip. Those copies complementary to the oligomers on the chip will bind strongly to the sites containing each of their complements.
After washing the chip, only the fluorescent complements will remain. A confocal scanner will interrogate each site. Those that generate light will indicate the presence of the oligomer associated with that site in the unknown sample. This technique can help researchers detect the presence of a cancer gene, as well as changes in the genes of the AIDS virus that would indicate a drug resistance, or the activation of a specific gene by a new drug candidate.
Until recently, all PMT applications were limited to between 100 and 1000 nm, but new photocathode materials have made it possible to detect phenomena out to 1700 nm. Singlet oxygen, for example, has a very weak, but long-duration emission at 1270 nm. It is believed to be one of the important reactants that causes photochemical damage. It is also the active agent in photodynamic cancer therapy.
In one application, researchers used 532-nm light from a pulsed laser to excite the dye Rose Bengal. Using a nuclear imaging resonance (NIR) PMT and a gated photon counter, the scientists were able to detect the delayed emission of the singlet oxygen formed when the excited triplet state of the Rose Bengal was quenched by ground-state oxygen.
This example and the others discussed are just a few of the applications that can benefit from the use of PMTs. All take advantage of the noise-free gain, many also require the high-speed detection and large active areas possible with these detectors.