Electron-multiplying CCDs remove low-light barriers
Electron-multiplying CCD technology-designed into a high-end, digital, cooled camera
Trends in photon measurement are placing unprecedented demands on detector technology to perform at increasingly higher levels of sensitivity. Electron-multiplying charge-coupled-device (EMCCD) technology is designed to respond to this growing need and open up new avenues of experimental imaging and spectroscopy. Low-concentration analysis of biological reactions, for example, can be carried out with minute samples that would otherwise be insufficient for measurement purposes.
Electron-multiplying CCD technology also allows tracking of the behavior and interactions of individual biomolecules at exceptionally rapid frame rates, even within their natural cellular environment. Other techniques, such as intracellular ion-signaling microscopy (like calcium-flux microscopy) and multidimensional microscopy on live cells, impose considerable demands on detection technology because of the need for higher sensitivity at fast frame speeds.
Similarly, areas in physical sciences such as Raman spectroscopy, Bose-Einstein condensation, astronomy (including adaptive optics), and neutron tomography are benefiting from enhanced sensitivity at shorter exposure times.
EMCCD technology brief
Sometimes known as “on-chip multiplication,” EMCCD technology is an innovation first introduced to the digital scientific-imaging community by Andor Technology in 2001.
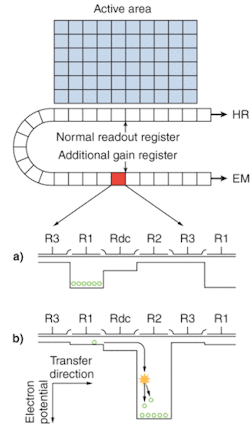
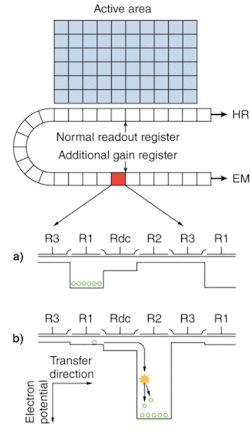
Electron-multiplying CCD sensors are manufactured utilizing standard CCD fabrication techniques. The unique feature of the EMCCD sensor is the electron-multiplying structure that is inserted between the end of the shift register and the output amplifier, referred to as the gain register (see Fig. 1a). This structure is similar to the shift register except that the R2 phase (of the usual three phases, R1, R2, and R3; see Fig. 1b) of this section is replaced with two electrodes, the first held at a fixed potential and the second clocked as normal, except that a much higher voltage amplitude is used (between 40 and 50 V) than is necessary for charge transfer alone.
The design of the fixed-voltage electrode, the clocked electrode, and the relatively large voltage difference between them, results in an intense electric field that is sufficiently high for the transferring electrons to undergo impact ionization. Impact ionization generates new electrons that lead to signal multiplication or gain. The probability of multiplication per transfer is actually quite small, only around 1.01× to 1.015× at most. This may seem insignificant, but when executed over a large number of transfers, a substantial gain is achieved.
Inserting the electron amplification stage before the output amplifier allows the signal to be increased above the readout noise, hence effectively reducing the readout noise. In fact, a gain level equal to the readout noise in electrons should reduce the effective noise to one electron, and higher gains will result in sub-one-electron values, which for any practical purposes means that readout noise can be ignored. More important, by using higher EMCCD gains at higher readout speeds, this noise performance can be achieved at any frame rate.
Applications
Electron-multiplying CCD technology is particularly suited to optical configurations that are designed to minimize background photon levels, thus placing more emphasis on the detector as the true experimental detection limit. The EMCCD is a common choice for single-molecule detection experiments, for example, typically using total-internal-reflectance fluorescence microscopy for parallel studies of single-molecule dynamics under reduced excitation power. The ability to detect single-molecules in such a fashion has catalyzed the race to develop a method that can sequence the entire human genome in less than 24 hours.
Live-cell fluorescence microscopy (including calcium-flux, time-lapse, cell-motility, and four- and five-dimensional confocal microscopy) has advanced enormously from this ultrasensitive EMCCD technology, reaping the benefits of improved temporal and spatial resolution, and minimization of cell phototoxicity and dye photobleaching due to allowed reductions in light exposure and application of dyes into the cell structure. An imaging rate of more than 30 sections through a cell per second has recently been demonstrated in Derek Toomre’s “CINEMA” lab at the Department of Cell Biology, Yale University (New Haven, CT), using Andor iQ multidimensional microscopy software, which is optimized for the iXon EMCCD camera used in these experiments. The software maintains tight synchronization between the camera, a piezoelectric translation stage, and a fast-switchable light source, to deliver optimal exposure and acquisition overlap. Camera sensitivity allows the use of shorter exposure times, while rapid synchronous shuttering makes possible higher temporal resolution through multiple dimensions, while at the same time allowing capture of more images before cells succumb to phototoxicity.
Experiments such as calcium-flux microscopy require significantly lower fluorophore dye concentrations, resulting in reduced buffering of free calcium (Ca2+) by calcium-binding dye. Previously, Mark Hollywood of the Smooth Muscle Research Group at the Queen’s University (Belfast, Northern Ireland) had to use a high concentration of Fluo-4 calcium-binding dye to reach sufficient signal intensity with a standard camera, and the calcium wave (a wave is a propagation of calcium through the cell and central to cellular function in many tissues) only partially traversed the cell length. The EMCCD’s ability to capture signal with reduced dye concentrations allowed the group to capture the calcium wave spreading across the full length of a smooth-muscle cell (see Fig. 2).
Of particular suitability to EMCCD technology are inherently low-light optical configurations. Prime examples of this are the spinning-disk-based confocal microscopes, such as the laser/microlens system from Yokogawa Electric (Tokyo, Japan), or the CARV filtered white-light confocal imaging system from Atto Bioscience (Rockville, MD; now part of BD Biosciences). The spinning disk, because of its confocal nature and potentially rapid frame rates (up to 1000 full confocal images per second is possible), reduces fluorescence signal levels to the regime of from 1 to 20 photons/pixel/s. In addition to electron-multiplying requirements, the camera’s frame rate must be able to synchronize with the spinning-disk frame rate to avoid distinctive banding patterns in the images.
The EMCCD is also suited to detecting intracellular bio- and chemiluminescence. These applications use no excitation source and so harness the true single-photon-sensitive nature of the detector without fear of masking the signal by background photons. Under such circumstances cooling becomes critically important, and if photon flux is extremely low, high signal-to-noise images can be obtained through use of longer exposure times.
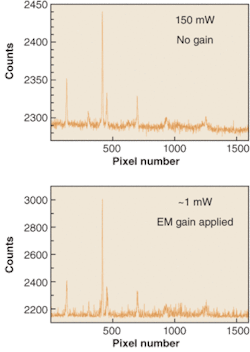
The latest addition to EM technology comes in the form of exclusive EMCCD cameras for spectroscopy. The spectra of an acetonitrile/toluene mixture have been recorded with significantly different laser powers by an Andor Newton EMCCD camera (see Fig. 3). This camera houses a vacuum-sealed, deep-cooled back-illuminated 1600 × 400 sensor. Electron-multiplying gain has been applied to the lower power spectrum to achieve a very similar signal-to-noise ratio as that evident in the higher-power spectrum in the absence of EM gain.
Optimizing EMCCDs
To effectively harness EMCCD technology, several key parameters must be optimized during camera design. The iXon (imaging) and Newton (spectroscopy) cameras were designed from first principles to maximize the potential of EMCCD technology.
One area that requires particular attention, for example, is thermo-electric (TE) cooling. By housing the sensor in a hermetically sealed, permanent vacuum enclosure, designed also for minimal outgassing (rather than the lower-end approach of an o-ring sealed, gas-filled housing) it is possible to achieve significantly lower temperatures in any ambient condition (down to -100°C). Enhanced cooling performance is a critical requirement in EMCCD technology because thermally generated electrons are amplified above the read-noise floor, just as photoelectrons will be. Another significant advantage of a permanent vacuum head is the protection it gives the sensor from moisture and volatile condensates. In fact, this approach enables removal of a window from the front of the sensor that is otherwise required for protection, resulting in increased throughput of the signal from a one-window design. And removing convection from the sensor enclosure also eliminates the possibility of condensation on the outside of the front window.
Another important aspect of EMCCD performance is charge shifting. It is beneficial in a frame-transfer device to maximize vertical clock times, thereby minimizing smearing under short exposure times and increasing frame rate. The challenge comes in doing this while retaining the essential sensitivity performance, since a form of spurious single-electron noise called clock-induced charge is particularly sensitive to clock conditions. An EMCCD can be operated successfully under these more challenging conditions if the voltage clocking parameters are optimized.
Sometimes, relatively minor specification details can make a discernable difference to performance. Atom Sarkar of Columbia University (New York, NY), for instance, has been investigating protein unfolding through the z-direction of a total-internal-reflection-fluorescence (TIRF) evanescent field (an evanescent field decays exponentially into the sample from the surface, covering distances typically of a few hundred nanometers and yielding a very narrow plane of excitation). By combining TIRF with atomic-force microscopy (AFM), distance calibration through the TIRF field made photonic-based evanescent nanometry possible. The arrangement required minimal vibration to prevent distortions in the AFM cantilever response so the ability to switch off the fan of the iXon EMCCD during measurement periods was critical.
The unique capabilities of EMCCD technology have resulted in its rapid acceptance across an ever-expanding range of applications. This trend will likely continue unless more-sensitive technology comes along.❏
OLIVIER BERNARD is market development manager for spectroscopy and COLIN COATES is market development manager for bioimaging at Andor Technology, 7 Millenium Way, Springvale Business Park, Belfast BT12 7AL, Northern Ireland; e-mail: [email protected], [email protected].