Ultrafast-laser ablation of polymer multicore fibers enhances biomedical sensing
HARIKUMAR K. CHANDRASEKHARAN, EUNAN P. McSHANE, KEVIN DHALIWAL, MICHAEL G. TANNER, and ROBERT R. THOMSON
Advances in fiber-optic technologies have revolutionized many areas, including biomedicine and healthcare. Today, optical fibers are a mainstream component in modern bespoke medical devices enabling efficient diagnosis, treatment, and monitoring. However, despite their valuable contribution in novel biosensors, there is an urgent push to enhance their light delivery and collection capabilities for in vivo sensing applications.
While most fiber-based sensors and imaging probes are based on conventional glass fibers, there is a growing interest in polymer optical fibers (POFs) for low-cost sensor applications. Their inherent elasticity and ability to detect physical parameters such as high dynamic strain, temperature, and pressure make them an ideal alternative to conventional glass optical fibers.
With the goal of developing low-cost fiber-optic sensing probes that are versatile, compact, and capable of distributed sensing, ultrafast laser pulses can be used to ablate the side of a multicore POF. This allows different cores of the POF to emit/collect light at different positions along the POF. Such a laser-modified POF can potentially act as send-and-receiver type elements in novel biosensors, enabling multipoint light delivery and collection from the surrounding environment at desired locations with high illumination density.1
Femtosecond laser micromachining
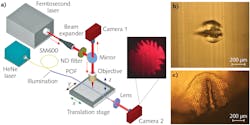
Ultrafast laser micromachining, an established technology in modern microprocessing to produce microscale features in materials, was used to ablate the POF. As shown in Figure 1a, femtosecond laser pulses (360 fs pulse duration) at a central wavelength of 1030 nm were used to ablate selected regions of a highly multicore POF. The fiber was attached to a high-precision 3D translation stage enabling precise movement of the POF with high accuracy and control. The approximately 0.54 μJ pulses were tightly focused on the POF using an objective lens. Due to the short duration of the pulses, the peak intensity of the focused spot on the fiber surface was sufficiently high to induce phase transformation, which leads to ablation and the eventual formation of an ablated trench, as shown in Figures 1b and 1c.
The formation of the microstructures by ultrafast laser ablation can be viewed and controlled effectively in the experiment. For this, the proximal end of the POF was flood illuminated with a fiber-coupled HeNe laser and the distal end imaged onto a CMOS camera (Camera 2). As the cores in the POF are closely packed, the laser modification results in removal of a group of cores forming a conical-like structure as shown in Figure 1c, which makes the emission characteristics highly sensitive to input light launching.
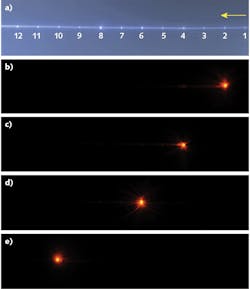
If performed correctly, the precision ablation of the POF enabled selection and control of the emission profile at discrete points along the fiber. The choice of individual emission point through selective core illumination is a unique capability of this laser-modified fiber. To characterize the emission points, light at a 780 nm wavelength was butt-coupled to the POF using a single-mode fiber (SMF), which can be translated across the POF facet in x-y-z directions. A CCD camera was then used to image the entire section of the fiber with 12 microstructures and to optimize the light coupling in each of them. The CCD images of the fiber with 12 emission points (see Fig. 2a) obtained by proximal-end all-core flood illumination and four side-emitting fiber points along the fiber (see Figs. 2b-e) with selection by butt-coupling are shown.Another crucial characteristic of the laser-modified fiber point is the throughput—the fraction of input light that exits from the fiber at the position of the ablated microstructure. Using a fiber-cutback method, the measured throughputs of the fabricated microstructures range from 55% to 73%, which are the highest achievable throughputs for a side-firing fiber reported so far. A future fabrication system would aim to improve the throughputs of the emission points with optimum fiber and fabrication parameters.
Biomedical potential
The next step is to integrate the multipoint-emission fiber, with optimization, in devices to perform a range of biomedical applications such as fluorescence,2 confocal imaging,3 photobiomodulation,4 optical tomography,5 medical device location,6 and 3D optical coherence tomography (OCT).7 The discrete emission of light has potential in medical diagnostics, enabling spectroscopic investigation at many points along a medical device throughout an organ or extended tissue structures. During medical-device placement—for instance, central venous catheter placement—multipoint light emission along the device length could be viewed through the skin.
An immediate application of such a fiber is in medical-device location identification deep inside the body, where light must transit through significant scattering biological media. Here, the discrete sources of light act as known points that can be located serially in combination with time-resolved imaging techniques. In in vivo 3D OCT, multiple custom-built platforms can be integrated with the emission points within the fiber; these platforms consist of microlens-prism combinations for automated 3D illumination to the target and collection of signals to the detectors. The future low-cost multipoint fiber probe would potentially replace some of the existing sensors in minimally invasive surgery, especially in medical devices that require single-use disposable catheters and endoscopes.
ACKNOWLEDGEMENT
The authors thank Asahi Kasei Corporation (Tokyo, Japan) for providing the PMMA imaging fiber, and for support from the UK Science and Technology Facilities Council (ST/S000763/1).
REFERENCES
1. H. K. Chandrasekharan et al., OSA Technical Digest, paper TM2B.5 (2020); https://doi.org/10.1364/translational.2020.tm2b.5.
2. M. Hirano et al., Brain Res., 732, 61–68 (1996).
3. W. Gobel et al., Opt. Lett., 29, 2521–2523 (2004).
4. R. Zomorrodi et al., Sci. Rep., 9, 6309 (2019).
5. S. Mosca et al., Biomed. Opt. Express, 11, 1697–1706 (2020).
6. M. G. Tanner et al., Biomed. Opt. Express, 8, 3 (2017).
7. H. Pahlevaninezhad et al., Nat. Photonics, 12, 540–547 (2018).
Harikumar K. Chandrasekharan (1), Eunan P. McShane (1, 2), Kevin Dhaliwal (2), Michael G. Tanner (1, 2), and Robert R. Thomson (1, 2) are from (1) Scottish Universities Physics Alliance (SUPA), Institute of Photonics and Quantum Sciences at Heriot-Watt University and (2) the Centre for Inflammation Research, Queen’s Medical Research Institute, University of Edinburgh, both in Edinburgh, Scotland; e-mail: [email protected].