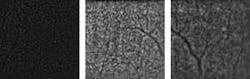
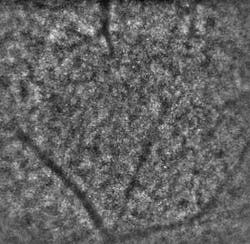
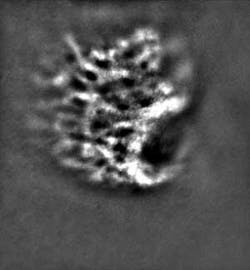
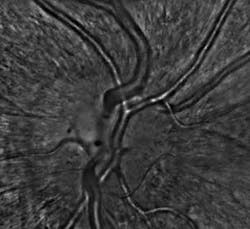
A recently developed prototype adaptive-optics system has been shown to image the eye at the cellular level. The system, which is of practical size for use in a physician’s office or clinic, may result in much earlier diagnoses of a wide variety of diseases that severely impair vision and cause blindness.
Eyesight can be lost or altered due to any number of pathologies ranging from proliferative diabetic retinopathy, glaucoma or the increasingly prevalent age-related macular degeneration (AMD) on to the far less common, but equally handicapping, retinitis pigmentosa or Usher syndrome. In virtually every case, the key to saving patients’ vision lies in early diagnosis and timely treatment.
Most retinal diseases primarily affect microstructures that are largely invisible to fundus cameras and ophthalmoscopes. Even in high-performance optical-coherence-tomography (OCT) systems and scanning laser ophthalmoscopes (SLO), imperfections in the eye’s optical system induce blur that limits lateral resolution and renders structures just a few microns in size indistinguishable.
Many scientists have worked to augment current technologies with adaptive optics, but while they have produced some impressive results, laboratory prototypes have been unsuitable for use in clinical research. They typically require a large amount of space and significant technical expertise beyond that of most practicing ophthalmologists. Also, most have very small imaging fields and thus must acquire numerous adjacent images in order to construct an image of usable size. Finally, the corrective ability of their adaptive-optics components, in particular their deformable mirrors, is too narrow to compensate for the wide variety of optical defects found in human eyes.
Field testing a clinical design
The INOVEO project began in 2006 to overcome these limitations. It was initiated by a consortium1 of 11 French academic, industrial, and medical experts, and is coordinated by Imagine Eyes (Orsay, France), a commercial developer of advanced ophthalmic medical devices based on adaptive-optics technologies. INOVEO received €1.2 million (US$1.5 million) in grants from the French ANR (Agence Nationale pour la Recherche) and the Retina France association. In addition, participating industrial members contributed their own internal resources.
Specifically, the project’s primary objective was to develop an adaptive-optics system capable of imaging the retina at the cellular level. As a prototype, the system would enable clinical investigations to prove its medical utility.
Just recently, the consortium delivered two of three planned prototypes of its Adaptive Optics Flood Illumination Fundus Camera (AO FIFC) for field tests to evaluate their potential for clinical investigations. Initial feedback from the consortium’s medical partners has been positive. “When the first INOVEO prototype reached our hospital, we were immediately startled by its compact size, similar to that of a standard retinal camera,” notes Olivier Roch, MD, at the Hôpital Necker, a children’s hospital in Paris. “While the adaptive-optics systems that we had previously seen in various publications seemed to occupy the surface of a very large desk, the new prototype fit perfectly into our clinical setting. After just a couple of days of training, our medical researchers were able to immediately proceed with the project’s clinical investigation program. The first cellular-resolution images revealed an impressive level of detail that could never have been seen with conventional imaging instruments.”
Specs and operation
“A major challenge in the INOVEO project was the need to compensate for the wide-ranging optical defects that exist in human eyes. Existing deformable mirrors had a stroke of less than 10 µm and therefore could not assume the shapes needed to correct the deviations found in eyes suffering from significant astigmatism and irregular optical imperfections,” notes Fabrice Harms, R&D manager at Imagine Eyes. “Researchers at the LAOG (Laboratoire d’Astrophysique de Grenoble), members of the INOVEO consortium, overcame that limitation when they invented mirao, a completely new deformable mirror technology that uses miniature electromagnetic actuators to deform a thin reflective membrane. mirao’s 50 µm stroke enables it to correct for extreme ocular optical defects while using very low input voltage–a top priority for safely integrating technologies into medical systems.”
Inside the AO FIFC is a Shack-Hartmann sensor with 1024 lenslets (HASO 32-eye) that measures the angular deviations of light rays passing through the patient’s eye, a large-stroke 52-actuator electromagnetic deformable mirror (mirao 52-e) that compensates for the measured deviations, and a high-resolution/low-noise CCD camera for image acquisition. A modified Badal assembly compensates for large defocus errors. An internal OLED microdisplay acts as a fixation target for the patient, while an infrared superluminescent diode (SLD) functioning at 750 nm provides a source for the Shack-Hartmann sensor; and a specially designed infrared flood illumination system, functioning at 850 nm, provides consistent lighting throughout the area of the retina to be imaged. A standard PC running Windows is used to control the device.
The system is designed so that a patient sits in front of it with his or her head positioned on a standard ophthalmic chinrest (see Fig. 1). The doctor performing the imaging procedure easily aligns the device with the patient’s eye and determines the retinal area to be imaged. The patient’s fixation is stimulated by a selectable target image displayed on the internal microdisplay.
The fact that the Shack-Hartmann sensor’s source and the imaging diodes both use infrared light is an important advantage. Because the source is invisible to patients, the system does not induce the blinking reflex that is associated with the visible light flashes used in some techniques. Blinking causes eye movement and thus severely degrades image quality.A specially designed, high-accuracy pupil tracking system (PTS) is currently being developed for incorporation into the AO FIFC prototypes. This addition will further enhance both the temporal performance of the adaptive-optics system and the device’s ability to acquire sequential images for mosaicing. The PTS tracks the center of the pupil with an accuracy of better than 15 µm in a ±2 mm range of eye movement. The software that drives the PTS processes video images at approximately 90 Hz and feeds the pupil position data back to the adaptive-optics control algorithms at the same rate.
Going forward
Different pathologies manifest themselves in diverse manners at the microscopic level, and doctors and researchers alike need tools that provide versatile features that will enable them to diagnose and treat the widest possible variety of patients.
“In cases of age-related macular degeneration (AMD) where the progression of the disease is characterized by a gradual loss of central vision, measuring the density of photoreceptors while observing drusens (lipoprotein deposits between the pigment epithelium and the Bruch membrane) may help improve the diagnosis of AMD and the prognosis for patients. Moreover, providing doctors with the ability to track signs of neovascularization will be a powerful tool in treatment planning and maintenance in their attempts to save the retina before significant damage occurs,” says Nathalie Massamba, MD, from the Centre Hospitalier Intercommunal de Créteil.
The same is true in the inverse sense for diabetic retinopathy, macular teleangiectasia (hypertensive retinopathy), and other vascular pathologies that are characterized by a loss of blood flow due to arterial and venous occlusions, which cause ischemia to various regions of the retina and results in tissue necrosis. The AO FIFC may help doctors detect minute fluctuations or abnormalities in blood circulation and intervene in time to avoid debilitating effects.
“The AO FIFC’s high resolution and relatively wide field of view make it an invaluable device for diagnosing macular holes when conventional imaging techniques are unable to confirm the patient’s subjectively reported visual symptoms,” notes Kiyoko Nakashima, MD, from the Hôpital Necker. The macula is responsible for our detailed, sharp vision and, at the early stages of this disorder, changes in visual acuity often occur or straight lines may appear wavy. Because the AO FIFC enables doctors to assess the presence and function of photoreceptors around the macular hole and monitor its two-dimensional progression, this may help them to decide whether a natural regression is likely or if a surgical intervention is required.
On the research front, adaptive-optics retinal imaging holds enormous promise. For example, we know the effects of chronic, or open-angle, glaucoma that is characterized by progressive death of the network of nerves that run over the retina’s surface and that is often accompanied by abnormal increases in intraocular pressure–yet we know very little about the origins of the disease. Providing researchers with the ability to image retinal microstructures, especially the lacrima cribosa in this case, may help them better understand the nature of the disease and work toward new sight-saving treatments.
“With regard to the rarer, but no less serious, retinal dystrophies such as retinitis pigmentosa and other hereditary pathologies, adaptive-optics retinal imaging holds a twofold promise,” says Dr. Nakashima. “These pathologies, often characterized by a progressive loss of function in photoreceptor cells, are difficult to diagnose at their earliest stages. Not only can adaptive optics enable doctors to classify the symptoms sooner, it may equally provide unique insight into the manner in which these diseases progress. This, in turn, may be particularly useful in the development of new gene therapies.”
The applications of adaptive optics in clinical ophthalmology have just begun to show their merit. In the hands of talented and ingenious doctors and researchers, the technology realistically could play a pivotal role in reducing the number of cases of blindness and improving the quality of life for millions of people around the world.
INOVEO consortium is made up of Centre Hospitalier Intercommunal de Créteil, Hôpital Necker, Imagine Eyes, Cell and Tissue Imaging Facility, Institut Curie, L’Institut de la Vision, Laboratoire d’Astrophysique de Grenoble (LAOG, CNRS-UJF), Laboratoire d’Etudes Spatiales en Instrumentation en Astrophysique (LESIA, CNRS – Observatoire de Paris, Laboratoire de Traitement et de Transport d’Information (L2TI, Université Paris XIII), Mauna Kea Technologies, Office National d’Etude et de Recherches Aérospatiales (ONERA), Quantel Medical
References
- M. Kennedy, “The Return of the Optical Transport Market: Video, IP Services Drive New Growth Initiatives,” Telecommunications Online (Feb. 27, 2007).
- C. Medford, “Fiber Diet for Telecom,” Red Herring (Aug. 14, 2007).
About the Author
Mark Zacharria
Mktg/Comms
Mark Zacharria is cofounder and senior consultant of Elucido Partners (Paris, France).