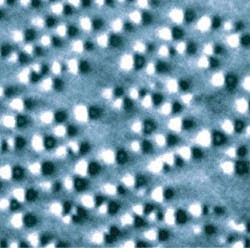
Biofriendly approach to quantum dots
Making good quantum dots for biological research is complex. But a process developed by NIST avoids a problematic step in the conventional approach, resulting in brighter, more stable dots. The traditional approach begins by inducing a semiconductor compound—typically a mixture of cadmium and selenium—to crystallize into discrete nanocrystals of just the right size. Cadmium is toxic and can oxidize easily (ruining the effect), so the nanocrystals must be encapsulated in a shell such as zinc sulfide. To make them water soluble for biological applications, a ligand is attached to the zinc atoms—and it serves as a tether to attach additional molecules that cause the dot to bind to specific proteins.
This method uses a high-temperature reaction that must be carefully controlled under an inert gas atmosphere. An intermediate ligand material that can tolerate the high temperature is used to promote the crystallization process, but it must be chemically swapped afterward for a different compound that makes the material water soluble. The ligand exchange step—as well as several variations on the process—is known to significantly alter the luminescence and stability of the resulting quantum dots.
Seeking a better method, NIST researchers turned to microwave-assisted chemistry. Working at temperatures half those of commercial processes, the group developed a relatively simple two-stage process that requires no special atmospheric conditions and directly incorporates the water-soluble ligand into the shell without an exchange step. Using commercially available starting materials, they have synthesized highly uniform and efficient quantum dots for a range of frequencies and shown them to be stable in aqueous solutions for longer than four months. The work is detailed in a paper, “Emission-Tunable Microwave Synthesis of Highly Luminescent Water Soluble CdSe/ZnS Quantum Dots.”
NIR, Raman spectroscopy move toward Parkinson’s diagnosis
A study published in Biomarkers in Medicine demonstrates proof-of-concept for use of minimally invasive technology to diagnose Parkinson’s disease (PD). Researchers used near-infrared (NIR) and Raman spectroscopy (RS) to develop a metabolic profile of biological markers for PD. The technology is being developed by Molecular Biometrics (Chester, NJ)—which has received an award from The Michael J. Fox Foundation for Parkinson’s Research to support further development and validate its methodology.
The study involved 52 patients, 20 with mild or moderate stages of Parkinson’s disease and 32 age-matched control subjects at Sir Mortimer B. Davis – Jewish General Hospital. Whole blood samples were analyzed using NIR and Raman spectroscopy methods to create metabolomic profiles of human biofluids, including serum and whole blood. Both NIR and RS methods were used to measure the degree of oxidative stress (OS) present in each sample. In differentiating Parkinson’s disease patients from the control group, RS achieved a sensitivity of 74% and specificity of 72%, with eight false positives and four false negatives. NIR achieved a sensitivity of 74% and specificity of 76%, with four false positives and five false negatives.
Editor’s note: See more on Molecular Biometrics’ technology on p. 20.
Stretchable camera foreshadows artificial retina
The layout of a new high-performance, hemispherical camera uses stretchable optoelectronics to mimic operation of the human eye. Researchers at the University of Illinois and Northwestern University used an array of single-crystalline silicon detectors and electronics, configured in a stretchable, interconnected mesh, to create the device. The work opens new possibilities for advanced camera design and foreshadows artificial retinas similar in concept to those in the movie Terminator.
“Conformally wrapping surfaces with stretchable sheets of optoelectronics provides a practical route for integrating well-developed planar device technologies onto complex curvilinear objects,” said John Rogers, the Flory-Founder Chair Professor of Materials Science and Engineering at Illinois, and author of a paper describing the work, which was published in the journal Nature.
The camera’s design is based on that of the human eye, which has a simple, single-element lens and a hemispherical detector. To make it, the researchers molded a thin rubber membrane in the shape of a hemisphere, which they stretched with a specialized mechanical stage to form a flat drumhead. Next, a prefabricated focal-plane array and associated electronics—created by conventional planar processing—were transferred from a silicon wafer to the tensioned, drumhead membrane.
When the tension was released, the membrane returned to its original shape. This process compressed the focal-plane array, causing specially designed electrical interconnects to delaminate from the rubber surface and form arcs, pinned on the ends by detector pixels. These deformations accommodate strains associated with the planar to hemispherical transformation, without stressing the silicon, as confirmed by mechanics modeling performed by researchers at Northwestern. The array package was then transfer-printed to a matching hemispherical glass substrate. Attaching a lens and connecting the camera to external electronics completed the assembly.
“This approach allows us to put electronics in places where we couldn’t before,” Rogers said. “We can now, for the first time, move device design beyond the flatland constraints of conventional wafer-based systems.”
Tiny sensor illuminates spinal biomechanics
Researchers at two Canadian universities—the University of Victoria and the University of British Columbia—say they have developed a miniature fiber-Bragg-grating (FBG) sensor. Calling the FBG “the only optical, the smallest, and the most mechanically compliant disc pressure sensor,” the researchers say it has proven its ability to measure pressure within the spine.
The intervertebral disc (IVD) pressure sensor boasts pressure sensitivity seven times greater than that of a bare fiber, and a major diameter and sensing area of only 400 µm and 0.03 mm2, respectively. The researchers say their design is an improvement over other FBG pressure sensors that achieve increased sensitivity through mechanical amplification schemes—which typically result in major diameters and sensing lengths of many millimeters, they say. This size advantage makes it possible for the researchers’ sensor to be used between discs in the spinal column—even cervical and degenerated discs. Thus, the sensor is expected to enable measurements that could lead to new understanding of biomechanics. Such research could potentially lead to discoveries regarding back pain.
The team has validated its sensor by conducting IVD pressure measurements in the spines of pigs, and comparing the FBG measurements to those obtained using the current standard sensor for IVD pressure. The sensor’s sensitivity, as predicted using numerical models, matched with that measured experimentally. IVD pressure measurements showed excellent repeatability, too, the researchers say, and agreement with those obtained from the standard sensor.
The team’s research is described in the August issue of the Institute of Physics’ Measurement Science and Technology Journal.
NIR system highlights cancer for precision surgery
An imaging tool developed by Massachusetts researchers, the fluorescence-assisted resection and exploration (FLARE) system, aims to highlight cancerous tissue so surgeons can more easily and accurately detect and remove it without harming healthy tissue. FLARE consists of a near-infrared (NIR) imager, a video monitor, and a computer—and is in early clinical trials. The system shows particular promise for improving surgery for breast, prostate, and lung cancer, whose tumor boundaries can be difficult to track at advanced stages; it may also help cancer surgeons avoid cutting critical structures such as blood vessels and nerves.
“The system has no moving parts, uses LEDs instead of lasers for excitation, makes no contact with the patient, and is sterile,” says project director John Frangioni, M.D., Ph.D., of Beth Israel Deaconess Medical Center (BIDMC) in Boston and codirector of its Center for Imaging Technology and Molecular Diagnostics. FLARE also lets physicians control multiple viewing angles and different magnification levels through the use of a footswitch.
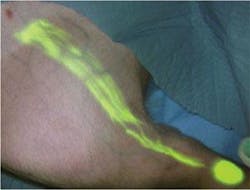
A pig’s hind leg is shown following injection with near-infrared contrast agents and imaging with near-infrared light. The imaging procedure highlights lymph flow.
“This technique is really the first time that cancer surgeons can see structures that are otherwise invisible, providing true image-guided surgery,” Frangioni says.
The system uses NIR fluorophores to target specific structures such as cancer cells when injected into patients. When exposed to NIR light, which is invisible to the human eye, the dyes or contrast agents light up the cancer cells and are shown on a video monitor. Images of these “glowing” cancer cells are then superimposed over images of the normal surgical field, allowing surgeons to easily see the cancer cells even in a background crowded by blood and other anatomical structures, the researcher says.
In preliminary studies, Frangioni and colleagues used the FLARE to successfully visualize organs and body fluids of mice and map the lymph nodes of pigs, all in real-time. The first human clinical trials, scheduled to be under way by the time you read this, involve mapping the lymph nodes of a small group of patients with breast cancer.