The U.S. Food and Drug Administration (FDA) recently approved the first clinical trial of quantum-dot technology in humans. The decision represents the first time the FDA has approved the use of inorganic material—in the same manner as a drug. The technology that will be tested was developed by researchers at Cornell University (Ithaca, NY), and is thus called Cornell dots or C dots. The trial will involve five melanoma patients at Memorial Sloan-Kettering Cancer Center (MSKCC) and will seek to verify that the dots are safe and effective.
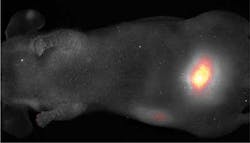
C dots are silica spheres less than 8 nm in diameter that enclose several dye molecules. The silica shell, essentially glass, is chemically inert and small enough to pass through the body and out in the urine. For clinical applications, the dots are coated with polyethylene glycol so the body will not recognize them as foreign. Organic molecules that bind to tumor surfaces—or even to specific locations within tumors—can be attached to the shell in order to make the dots stick to tumor cells. When exposed to near-infrared light, the dots fluoresce much brighter than unencapsulated dye, and thus identify the target cells.
The technology, the researchers say, can show the extent of a tumor's blood vessels, cell death, treatment response and invasive or metastatic spread to lymph nodes and distant organs. The safety and ability to be cleared from the body by the kidneys has been confirmed by studies in mice (Nano Letters, Vol. 9, No. 1). For the human trials, the dots will be labeled with radioactive iodine, which makes them visible in PET scans to show how many dots are taken up by tumors and where else in the body they go and for how long.
The dots provide a great advantage by remaining in the body long enough for surgery to be completed. In addition, they may serve as a carrier to deliver radioactivity or drugs to tumors. The original developers of C dots have founded a company, Hybrid Silica Technologies (Cambridge, MA), to commercialize the invention, which they say has many possible applications.
About the Author

Barbara Gefvert
Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.