Jaime Rodriguez-Canales and Jeffrey C. Hanson
Investigators have recently demonstrated that gene-expression profiles based on mRNA extracted from a whole tissue specimen differs markedly from the microdissected cells obtained from the same sample.1 That is why laser microdissection—the extraction of specific cell types from a histological sample—is becoming increasingly necessary. The approach enables accurate molecular profiling of specific cell populations in specimens.
Tissue samples obtained from patients represent the gold standard for defining the histopathological and molecular basis of disease. The cells on a histopathological slide contain the molecular alterations that characterize its unique morphology—and that enable diagnosis of a patient’s condition, and determine prognosis and therapeutic options.
Such specimens have an intricate three-dimensional architecture composed of diverse types of cell populations. For example, a breast-cancer biopsy typically contains normal and tumor epithelial cells, fibroblasts, adipose cells, blood vessels, and many types of inflammatory cells, all intermixed in a single slide. Because each phenotypically defined cell type has a particular molecular profile, analyzing them often requires that they be isolated.
A number of variations on the original laser-capture microdissection tool enable specific isolation of phenotypically defined cells. The approach has aided not only the discovery of novel molecular markers, but also, more recently, the study of the molecular alterations of the tumor microenvironment in cancer research. And it holds promise for more applications in the future.
Laser capture microdissection
The first positive-selection laser microdissection microscope, invented at the National Institutes of Health, resulted from a collaborative effort of a multidisciplinary team that included pathologists, physicists, and engineers.2 The goal in inventing the microscope was to isolate specific cells from a histological slide under a microscope.
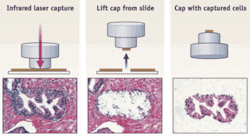
The original system, called laser-capture microdissection (LCM), is based on a near-infrared laser beam that activates a thermoplastic polymer (ethylene-vinyl acetate; EVA), which changes conformation. When the EVA polymer is placed on top of a tissue section, the IR-laser expands the EVA on top of the target cells and captures them without damaging the biomolecules (see Fig. 1). The polymer containing the captured cells can then be incubated in an appropriate extraction buffer to release and isolate the DNA, RNA, microRNA, or proteins for posterior molecular downstream analysis. The fact that this system requires tissue sections mounted on regular glass slides makes LCM very versatile and able to integrate into typical surgical pathology labs.
The initial laser-dissection system significantly impacted biomedical research. To date, there are more than 2,000 publications in the literature using laser microdissection, pointing to the important role that the technology now plays in various types of tissue analysis.
UV-laser and cutting systems
Shortly after the introduction of LCM, researchers began making variations on the original design. Many of these systems rely on high-energy ultraviolet (UV) lasers that cut out the target cells (when carefully done this does not damage the biomolecules). For the most part, the use of cutting lasers requires the tissue section to be mounted on special polyethylene naphthalate (PEN) membrane slides.
Today there are four types of systems that use UV-laser-based microdissection. The major differences among them relate to how they collect dissected cells.
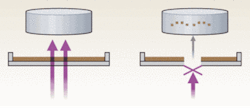
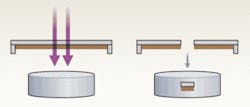
The elegantly designed laser-microdissection and pressure-catapulting technology used by P.A.L.M. Microlaser Technologies (a subsidiary of Carl Zeiss MicroImaging, Jena, Germany) differs from the original LCM in that it uses a noncontact method: a UV laser generates a photonic pressure that catapults the microdissected cells into a microcentrifuge tube cap (see Fig. 2). The PALM instrument is based on an inverted Zeiss microscope, which offers excellent optical capabilities. PALM is partially automated but is primarily operator-dependent because the researcher must identify and visually select cells for dissection.Another type of UV-laser cutting system is Molecular Machines & Industries’ (Glattbrugg, Switzerland) mmi CellCut, which retrieves the microdissected cells by adhesion without direct contact with the tissue sample. Its design avoids contamination by minimizing environmental contact during dissection.
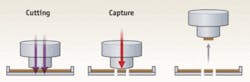
Finally, Molecular Devices (part of MDS Analytical Technologies; Toronto, ON, Canada) offers two semiautomated instruments—Veritas and Arcturus XT—that combine infrared and UV lasers. Veritas is a closed system without microscope oculars that allows capture and cutting microdissection with bright-light and fluorescence capabilities. More recently, the Arcturus XT was developed to add a better microscope platform—including contrast-phase, dark-field-like, and fluorescence illumination—along with a dual-laser approach. In both systems, the UV laser cuts around the cells of interest that are subsequently retrieved by capture into the EVA polymer with an infrared laser spot (see Fig. 4). With adequate sample preparation, these systems allow for precise and quick microdissections.Sample prep and analysis
One of the most important issues for microdissection is tissue processing. The majority of tissue specimens, especially clinical samples, are fixed in formalin and embedded in paraffin (FFPE). This procedure is considered the gold standard for diagnostic histopathology; however, it results in poor preservation of the biomolecules, especially mRNA and proteins. The ideal tissue preservation technique for molecular profiling studies is snap freezing in isopentane and liquid nitrogen.3 However, snap-frozen tissue specimens may be difficult to find except in limited numbers in some biorepositories and tissue banks. Moreover, snap-frozen samples produce poor histological quality and do not allow for efficient and low-cost storage of samples. Today, investigators are still in need of tissue preservatives that allow optimal histological quality, optimal molecular preservation, and long-term storage.
In any case, though, as soon as the tissue is on the histology slide, the cells need to be stained for microscopic identification and then microdissection. The most commonly used stains—hematoxylin-eosin (H&E), toluidine blue, and Nissl—are histochemical stains that allow excellent visualization of the cells without degradation of the biomolecules. To ensure that the staining does not damage the biomolecules, it is important to test the amount and integrity of the specific molecule to be analyzed, comparing a stained tissue section with the molecules extracted from an unstained control from the same specimen.
Immunohistochemistry (IHC) techniques, which use antibodies to label specific cells, are also available. These approaches, also called immuno-guided- or simply immuno-laser microdissection, enable identification of particular cell types, such as stem cells or components of the tumor microenvironment. The main caveat in their use is the effect on the yield and integrity of biomolecules—particularly RNA, because of the immunostaining procedure. In general, immunofluorescence (IF) techniques result in better preservation of bio-molecules in the tissue section than IHC.
Both IHC and IF work with the image-analysis software packaged with the latest versions of the dissection instruments. Such software can automatically identify the positively labeled cells and can aid the researcher performing microdissection. This modality allows for both high throughput and specific cell microdissection, particularly in samples in which the target cells cannot be identified using conventional histochemical techniques, such as cancer stem cells.
Finally, evaluation of the tissue slides by a pathologist is important because of the complexity of histological patterns. The pathology evaluation can help in the selection of the specimens for the study, the identification of the target cells, and the annotation of the areas to be microdissected.3 The pathologist also can provide advice on the tissue handling, microdissection techniques, and the potential clinical applications of the research project.
To enable evaluation following laser microdissection, the biomolecules can be easily extracted from the dissected cells using commercially available extraction kits. The samples should be evaluated for biomolecule concentration using spectrophotometers such as Nanodrop (Thermo Fisher Scientific, Waltham, MA), and for integrity using electrophoresis-based assays such as Bioanalyzer (Agilent Technologies, Santa Clara, CA).3 This is particularly important for RNA analysis. Polymerase chain reaction (PCR)-based assays also can be useful for the evaluation of amount and integrity of biomolecules in dissected samples.
Cancer insight and future directions
Laser microdissection has been widely used in biomedical research for the validation and discovery of novel molecular markers using tissue samples. In cancer research, laser microdissection has been used to characterize the molecular alterations that distinguish cancerous cells. More recently, one of the main focuses in cancer research has been the study of the molecular alterations of the tumor microenvironment, which is a highly complex network composed of cancer cells, fibroblasts, myofibroblasts, smooth muscle fibers, vessels, and inflammatory cells.
In the past, the stroma was thought to be primarily a passive support for epithelial cells. However, it has recently been found that the stroma around tumor cells has a very active role in carcinogenesis. For example, the stroma of the tumor regions in prostate cancer shares epigenetic alterations characteristic of cancer cells and are present in a distinct pattern.4 Additionally, it has been possible to identify alterations of gene expression in the tumor-associated stroma.5 These studies, enabled by laser microdissection technology, are bringing light into the epithelial-mesenchyma interactions that are important in the analysis of tumor progression and metastasis.
Future directions in microdissection techniques likely will include the development of high-throughput and automatic molecular-analysis techniques, improved methods for biomolecule preservation and amplification, and use of the technology in clinical practice in surgical pathology and oncology.
REFERENCES
- J. C. Harrell et. al., Clin. Exp. Metastasis 25(1) p. 81 (2008).
- M. R. Emmert-Buck et. al., Science 274(5289) p. 998 (Nov. 8, 1996).
- H.S. Erickson et. al., Nat. Protoc. 4(6) p. 902 (2009).
- J. Rodriguez-Canales et. al., J. Pathol. 211(4) p. 410 (March 2007).
- A.M. Richardson et. al., Diagn. Mol. Pathol. 16(4) p. 189 (Dec. 2007)
Jaime Rodriguez-Canales, M.D., is a research fellow/pathologist and Jeffrey C. Hanson, M.S., is biologist/biomedical engineer at the Laser Capture Microdissection Core Lab, Laboratory of Pathology, National Cancer Institute/National Institutes of Health, Bethesda, MD; home.ncifcrf.gov/ccr/lop/Research/lcm, e-mail: [email protected].